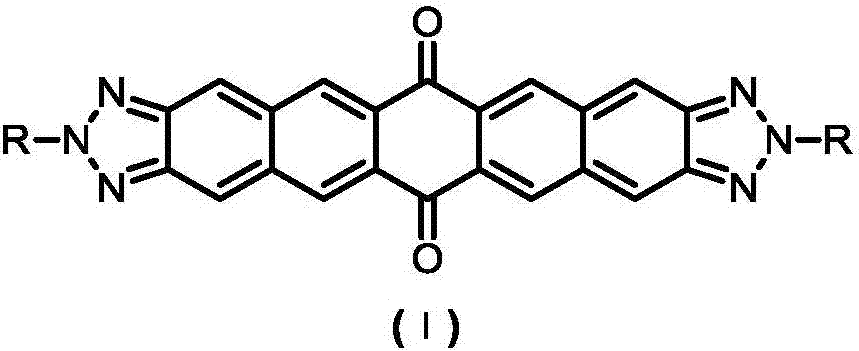
Bis-triazole pentacene quinone compound and preparation method thereof
A compound, the technology of pentabenzoquinone, which is applied in the field of chemical synthesis, can solve the problems of poor solubility and achieve the effect of simplifying the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
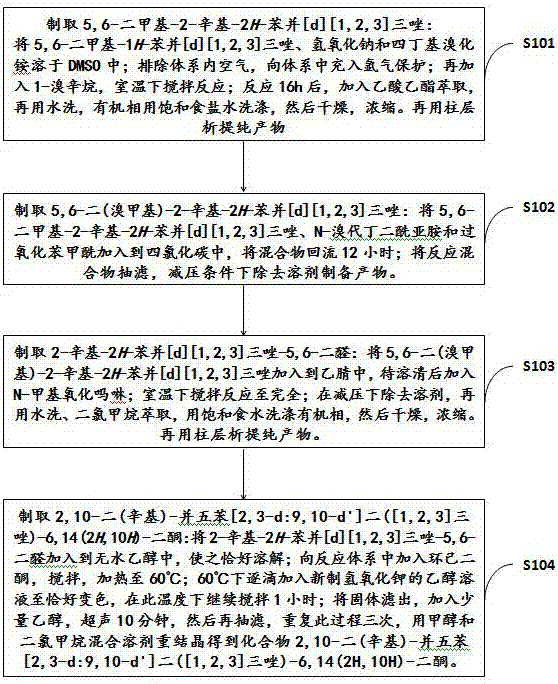
[0027] 2,10-dioctyl-pentacene[2,3-d:9,10-d']bis([1,2,3]triazole)-6,14(2H,10H)-dione ( A4)
[0028]
[0029] The preparation method of above-mentioned formula (A4) compound is as follows
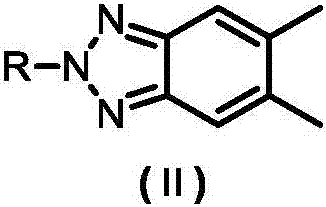
[0030] S101: Preparation of 5,6-dimethyl-2-octyl-2H-benzo[d][1,2,3]triazole (A1):
[0031]
[0032] 5,6-Dimethyl-2H-benzo[d][1,2,3]triazole (2.94 g, 20 mmol), sodium hydroxide (960 mg, 24 mmol) and catalytic amount of tetrabutylammonium bromide Dissolve in DMSO (250mL); remove the air in the reaction system, fill with argon; add 1-bromooctane (3.86g, 20mmol), and stir the reaction at room temperature; after reacting for 16 hours, add ethyl acetate to extract the mixture, It was washed with water again, and the organic phase was washed with saturated brine, then dried (anhydrous magnesium sulfate), and concentrated. The product was purified by column chromatography (petroleum ether / ethyl acetate, 15:1) to obtain product (A1) (2.07 g, 40%).
[0033] S102: Preparation of 5,6-bis(bromom...
Embodiment 2
[0043] 2,10-Dihexyl-pentacene[2,3-d:9,10-d']bis([1,2,3]triazole)-6,14(2H,10H)-dione (B4 )
[0044]
[0045] The preparation method of above-mentioned formula (B4) compound is as follows
[0046] S201: Preparation of 5,6-dimethyl-2-hexyl-2H-benzo[d][1,2,3]triazole (B1):
[0047]
[0048] 5,6-Dimethyl-2H-benzo[d][1,2,3]triazole (2.94 g, 20 mmol), sodium hydroxide (960 mg, 24 mmol) and catalytic amount of tetrabutylammonium bromide Dissolve in DMSO (250mL); remove the air in the reaction system, fill with argon; add 1-bromohexane (3.30g, 20mmol), and stir the reaction at room temperature; after reacting for 1 hour, add ethyl acetate to extract the mixture, It was washed with water again, and the organic phase was washed with saturated brine, then dried (anhydrous magnesium sulfate), and concentrated. The product was purified by column chromatography (petroleum ether / ethyl acetate, 15:1) to obtain product (B1) (1.94 g, 42%).
[0049] S202: Preparation of 5,6-bis(bromomet...
Embodiment 3
[0059] 2,10-Didecyl-pentacene[2,3-d:9,10-d']bis([1,2,3]triazole)-6,14(2H,10H)-dione ( C4)
[0060]
[0061] The preparation method of above-mentioned formula (C4) compound is as follows
[0062] S301: Preparation of 5,6-dimethyl-2-decyl-2H-benzo[d][1,2,3]triazole (C1):
[0063]
[0064] 5,6-Dimethyl-2H-benzo[d][1,2,3]triazole (2.94 g, 20 mmol), sodium hydroxide (960 mg, 24 mmol) and catalytic amount of tetrabutylammonium bromide Dissolve in DMSO (250mL); remove the air in the reaction system, fill with argon; add 1-bromodecane (4.42g, 20mmol), and stir the reaction at room temperature; after reacting for 16 hours, add ethyl acetate to extract the mixture, It was washed with water again, and the organic phase was washed with saturated brine, then dried (anhydrous magnesium sulfate), and concentrated. The product was purified by column chromatography (petroleum ether / ethyl acetate, 15:1) to obtain product (C1) (2.58 g, 45%).
[0065] S302: Preparation of 5,6-bis(bromom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com