RNA-protein-DNA in-situ multiple staining method of tissue chip
A tissue chip, -DNA technology, applied in the field of molecular biology, can solve the problem of not being able to detect and obtain biological information, and achieve the effect of reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 example
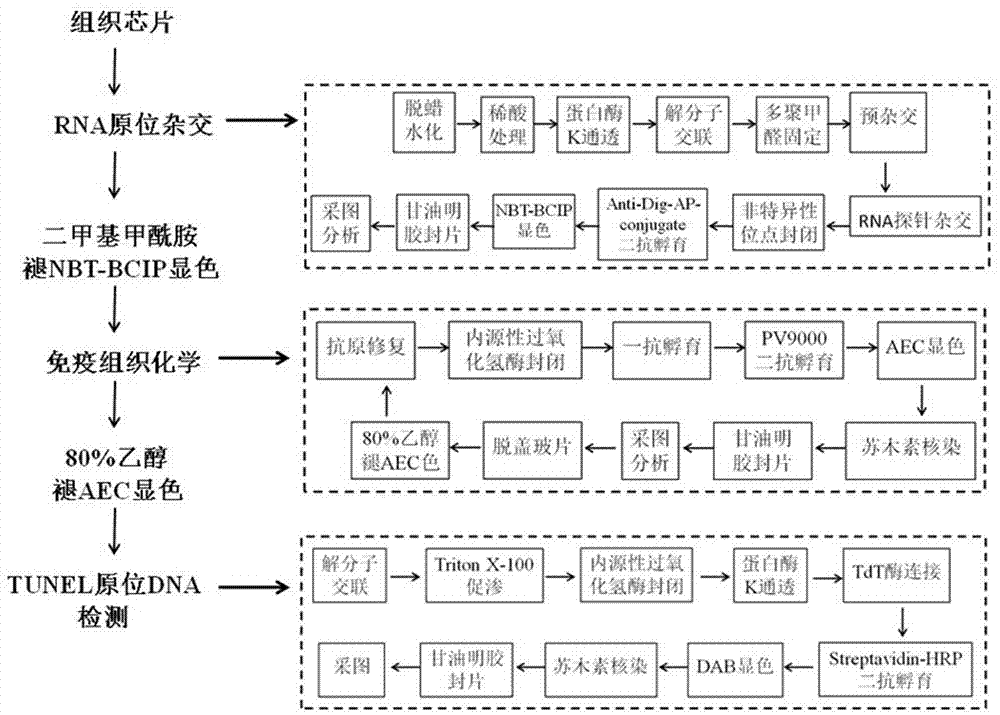
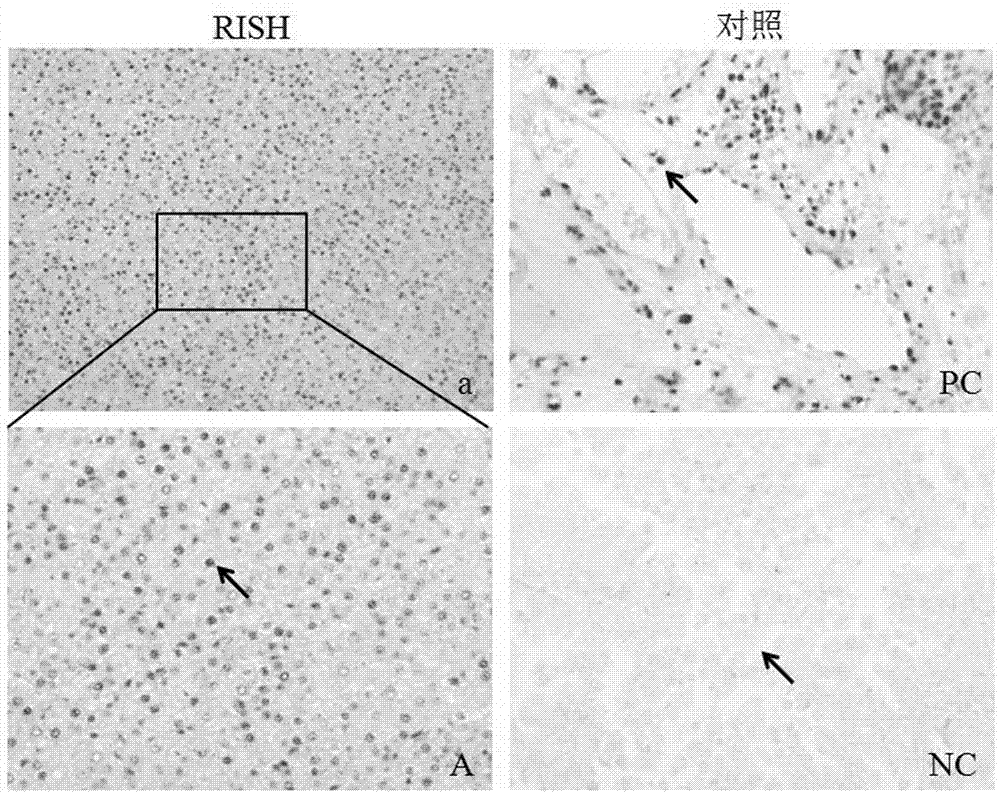
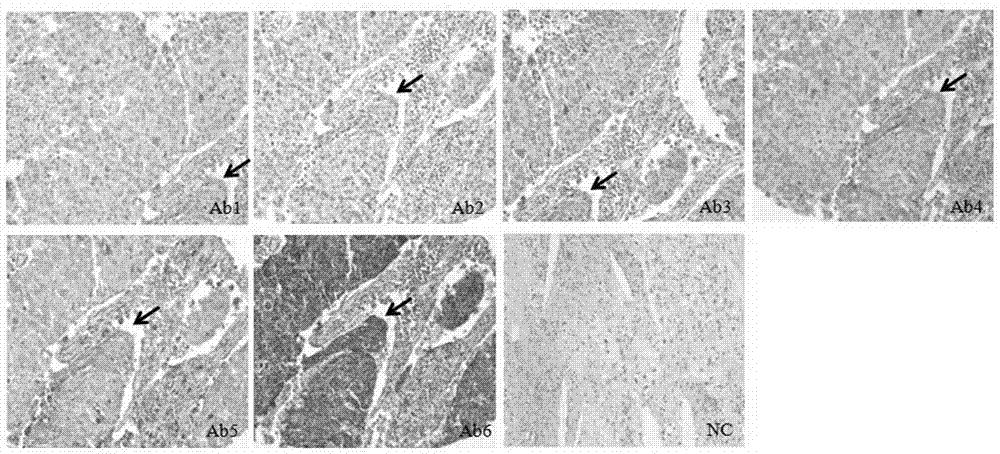
[0038] The present invention proposes a first embodiment, an RNA-protein-DNA in situ multiple staining method for a tissue chip, the method sequentially performs RNA in situ hybridization, immunohistochemical staining and DNA in situ hybridization on the same tissue chip;
[0039] In the process of RNA in situ hybridization, immunohistochemical staining and DNA in situ hybridization, an epitope antigen repair steamer is used to perform molecular cross-linking and unzipping or antigen repair on the tissue chip;
[0040] During RNA in situ hybridization and immunohistochemical staining, the slides were sealed with gelatin and glycerin after color development.
[0041] It should be noted that the epitope antigen retrieval steamer can effectively repair the "molecular mask" formed in the tissue section on the tissue chip fixed by paraformaldehyde, fully expose the antigenic determinant, and cross-link and dissolve with other molecules or Compared with antigen retrieval methods (su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com