Method for preparing gas diffusion electrode of hydrocarbon through electrochemical reduction of carbon dioxide
A gas diffusion electrode and hydrocarbon technology, applied in the direction of electrodes, electrode shape/type, electrolysis process, etc., can solve the problems of reducing the catalytic effect of catalysts, changing product distribution, poor stability, etc., and achieve low cost of raw materials, high Selectivity, anti-agglomeration effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
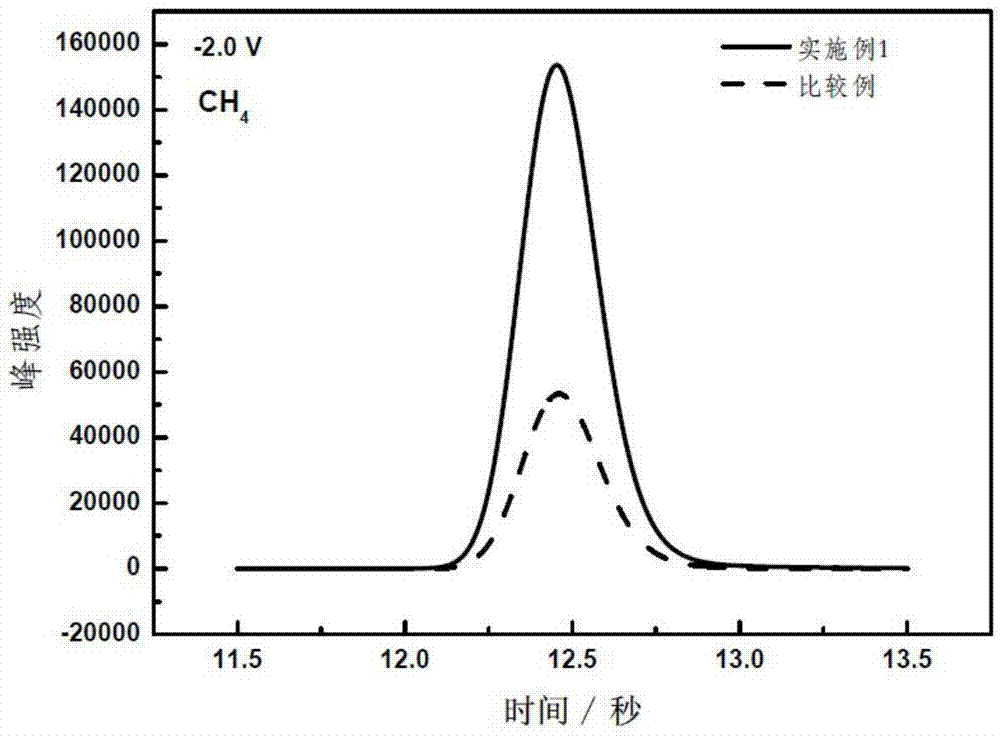
Embodiment 1
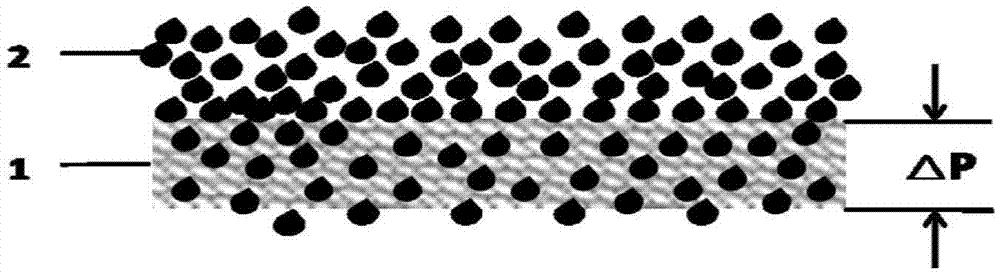
[0039] 1. Gas diffusion substrate heat treatment: Air is introduced into the tube furnace, the air flow rate is 100mlmin-1, and the area is 25cm 2 , TGP-H-060 carbon paper with a thickness of 0.2mm and a porosity of 78% was heat-treated at 500°C for 3 hours, then naturally cooled to room temperature, and the static water contact angle was measured to be 90°.
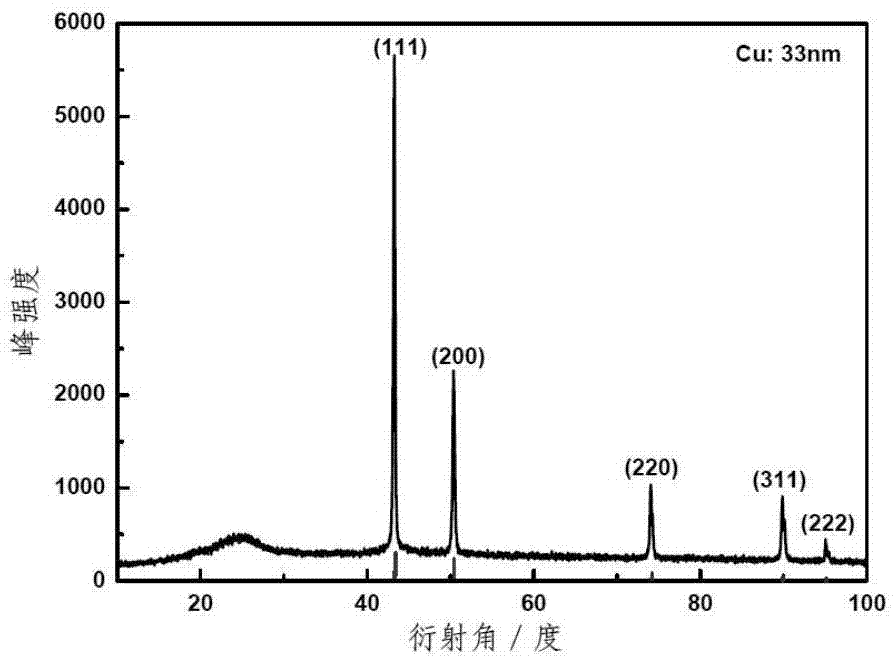
[0040] 2. Preparation of supported nano-Cu / C:
[0041] 1) Add 60ml deionized water and 170mg CuCl to a 250ml three-neck flask 2 2H 2 O, dissolved at room temperature;
[0042] 2) Dissolve 260mg of PVP with an average molecular weight of 45,000-58,000 in 40ml of deionized water, and transfer all of it to 1), and at the same time pass high Ar for gas replacement for 30min;
[0043] 3) 54mg KBH 4 Dissolve in 3ml deionized water, slowly add the aqueous solution dropwise to 2), control the dropping rate not higher than 0.5ml / min, the reaction system immediately appears wine red;
[0044] 4) According to the Cu:C mass ratio...
Embodiment 2
[0054] 1. Gas diffusion substrate heat treatment: Air is introduced into the tube furnace, the air flow rate is 100mlmin-1, and the area is 25cm 2 , TGP-H-030 carbon paper with a thickness of 0.1mm and a porosity of 82% was heat-treated at 300°C for 6 hours, and then naturally cooled to room temperature, and the static water contact angle was measured to be 130°.
[0055] 2. Preparation of supported nano-Cu / C:
[0056] 1) Add 39ml deionized water and 473mg Cu(NO3) to a 250ml three-necked flask 2 ·3H 2 O, dissolved at room temperature;
[0057] 2) Weigh 574 mg of trisodium citrate dihydrate, dissolve it in 5 ml of deionized water, and transfer all of it to 1), and at the same time pass high Ar for gas replacement for 30 min;
[0058] 3) 1.478g NaBH 4 Dissolve in 52ml of deionized water, slowly add the aqueous solution to 2) dropwise, control the dropping rate not higher than 1ml / min, the reaction system immediately appears wine red;
[0059] 4) According to the Cu:C mass r...
Embodiment 3
[0067] 1. Gas diffusion substrate heat treatment: Air is introduced into the tube furnace, the air flow rate is 100mlmin-1, and the area is 25cm 2 , a commercial carbon cloth with a thickness of 0.2mm and a porosity of 80% 2002HD was heat-treated at 600°C for 2 hours, then cooled down to room temperature naturally, and the static water contact angle was measured to be 60°.
[0068] 2. Preparation of supported nano-Cu / C:
[0069] 1) Add 7.8ml deionized water and 67mg CuCl to a 100ml three-neck flask 2 2H 2 O, dissolved at room temperature;
[0070] 2) Weigh 73 mg of disodium edetate dihydrate, dissolve it in 10 ml of deionized water, and transfer all of it to 1), and at the same time pass high Ar for gas replacement for 30 min;
[0071] 3) 106mg KBH 4 Dissolve in 6ml of deionized water, slowly add the aqueous solution dropwise to 2), control the dropping rate not higher than 0.2ml / min, the reaction system will slowly appear wine red;
[0072] 4) According to the Cu:C mas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com