Palbociclib crystalline compound and preparation method thereof
A compound and crystal form technology, which is applied in the field of palbociclib new crystal form compound and its preparation, can solve the problems of unguaranteed pharmaceutical crystal form and excessive ignition residue, so as to improve the quality of drug production, improve solubility and reaction The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Preparation of palbociclib crystal form compound
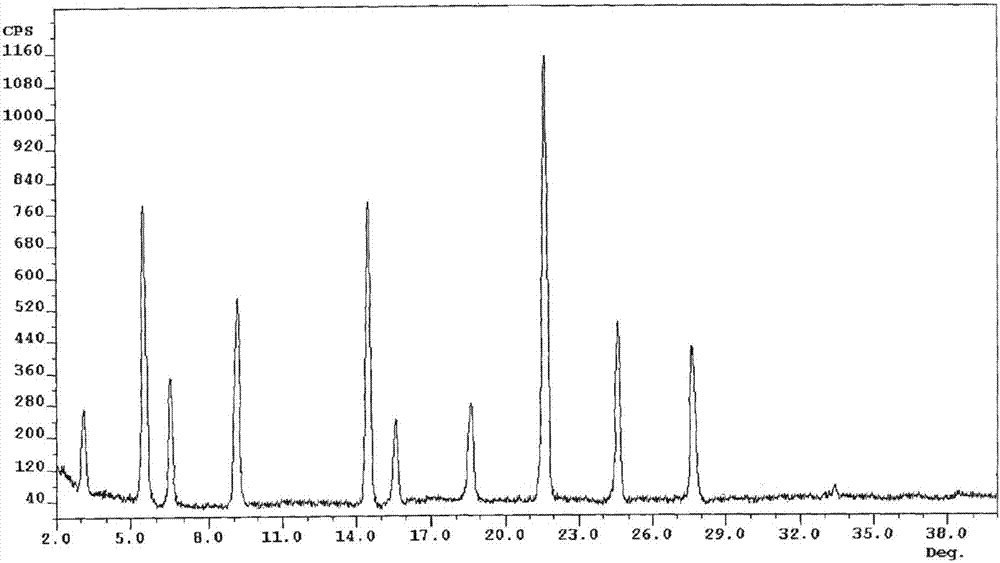
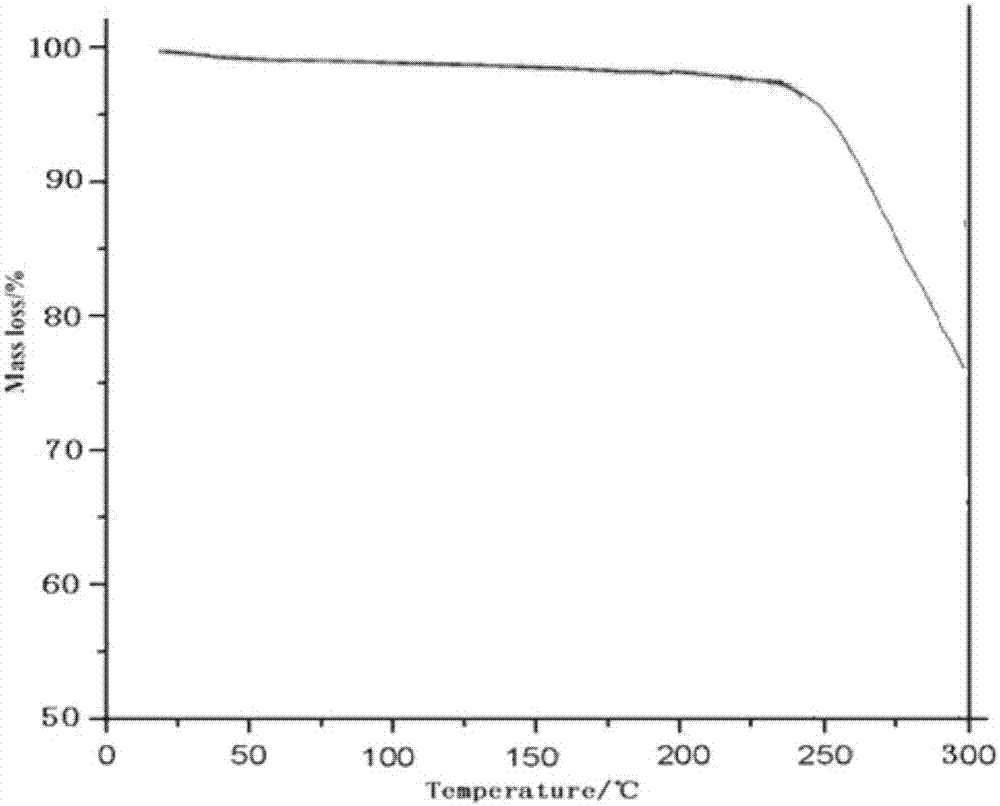
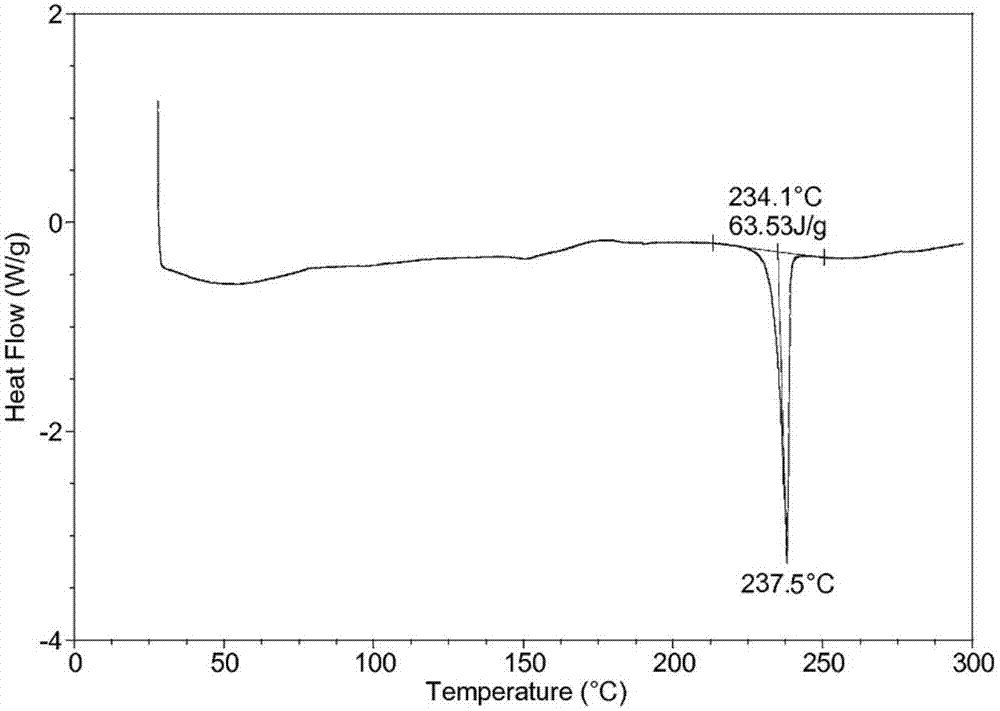
[0037]Take 50g of palbociclib in a reaction flask, add 1000ml of a mixed solution of dimethyl carbonate and ethanol (the volume ratio of dimethyl carbonate and ethanol is 3:1), heat to 70°C, stir to dissolve, and filter while hot ; While stirring, cool down to 20°C (the cooling range is 5°C per 10 minutes), add pre-cooled ethanol at a flow rate of 1.0mL / min to the solution (1000ml of ethanol is until the crystals come out, and continue to cool down to -5°C (cooling The amplitude is 1°C every 10 minutes), stirring for 3h. Vacuum suction filtration, the filter cake was vacuum-dried at 50°C for 6h to obtain 45.3g of light yellow solid with a yield of 90.5%.
Embodiment 2
[0038] Embodiment 2: Preparation of palbociclib crystal form compound
[0039] Take 50g of palbociclib in a reaction flask, add 1500ml of a mixed solution of dimethyl carbonate and ethanol (the volume ratio of dimethyl carbonate and ethanol is 2:1), heat to 75°C, stir to dissolve, and filter while hot While stirring, the temperature was lowered to 25°C (the cooling range was 5°C per 10 minutes), and 3000ml of pre-cooled ethanol was added to the solution at a flow rate of 1.5mL / min until crystallization, and the temperature was continued to drop to -10°C (the cooling range was 1°C every 10 minutes), stirring for 2h. After vacuum filtration, the filter cake was vacuum-dried at 50° C. for 4 hours to obtain 44.8 g of a light yellow solid with a yield of 89.6%. The X-ray powder diffraction spectrum of the obtained crystal measured by Cu-Kα rays is similar to that of Example 1.
Embodiment 3
[0040] Embodiment 3: Preparation of palbociclib crystal form compound
[0041] Take 50g of palbociclib in a reaction flask, add 2000ml of a mixed solution of dimethyl carbonate and ethanol (the volume ratio of dimethyl carbonate and ethanol is 2:1), heat to 65°C, stir to dissolve, and filter while hot While stirring, the temperature was lowered to 30° C. (the temperature drop was 5° C. per 10 minutes), and 2000 ml of pre-cooled ethanol was added to the solution at a flow rate of 2 mL / min to crystallization, and the temperature was continued to drop to -5° C. (the temperature drop was 5° C. per 10 minutes). 10 minutes at 1 ° C), stirred for 2h. After vacuum filtration, the filter cake was vacuum-dried at 50° C. for 5 h to obtain 45.7 g of a light yellow solid with a yield of 91.5%. The X-ray powder diffraction spectrum obtained by measuring the prepared palbociclib crystals using Cu-Kα rays is similar to that of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com