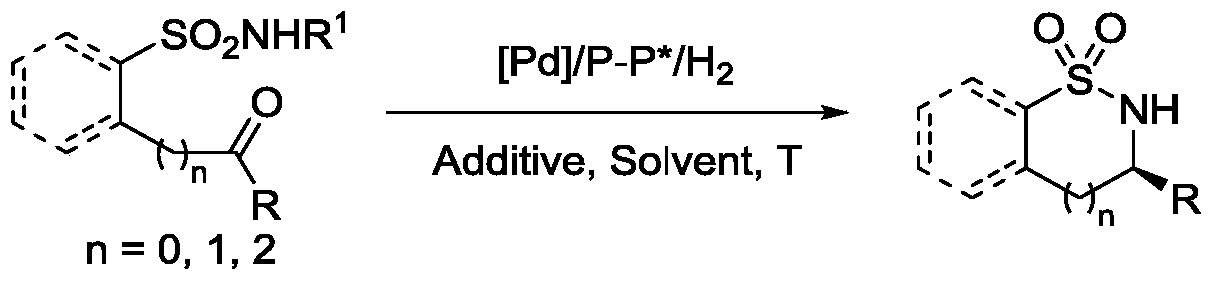
A method of a catalytic asymmetric molecule internal amine synthetic sulfa
An asymmetric, palladium-catalyzed technology, applied in the direction of organic chemistry, etc., to achieve the effect of simple and practical reaction operation, complete reaction and convenient separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
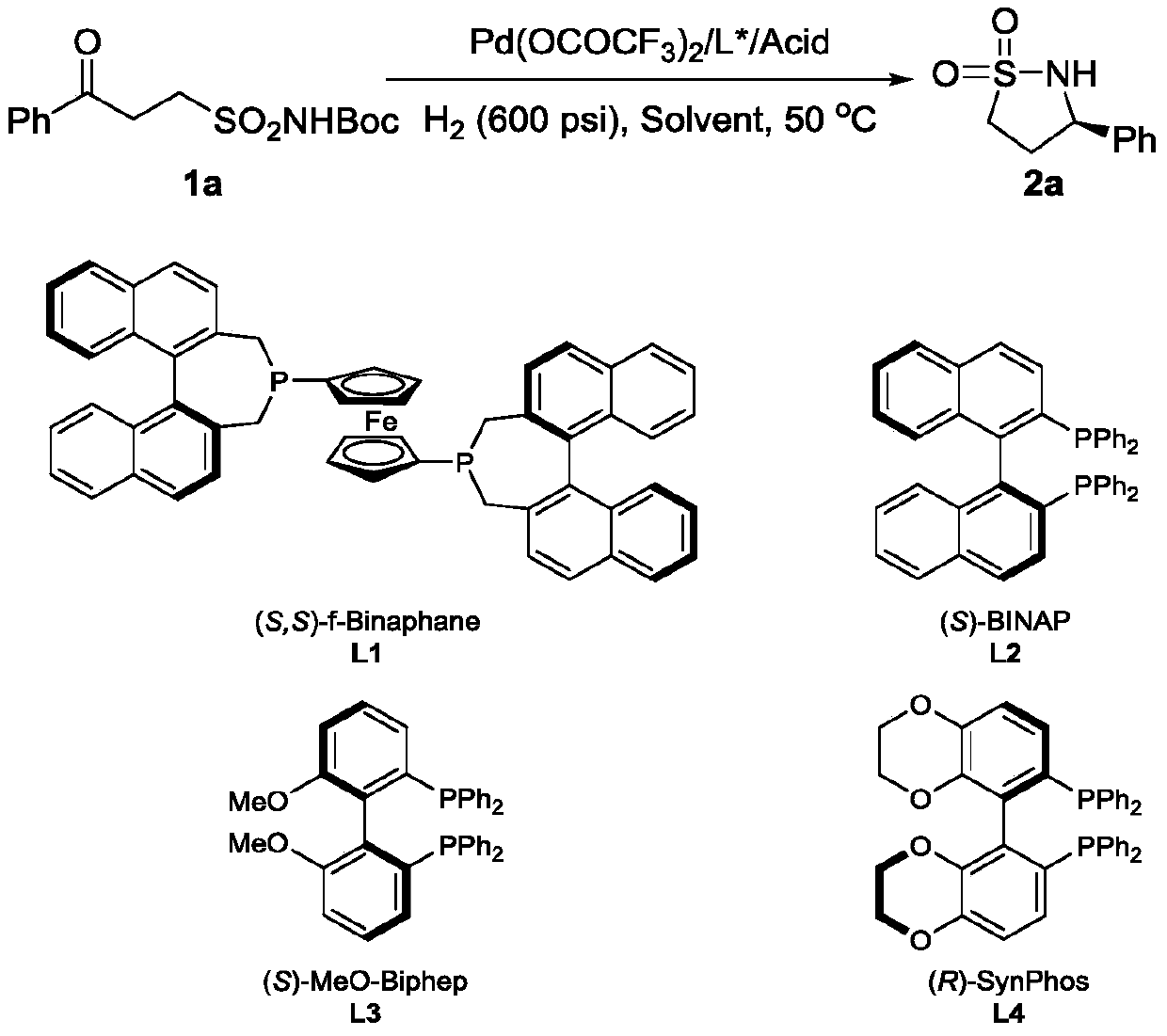
[0031] Embodiment 1: optimization of conditions
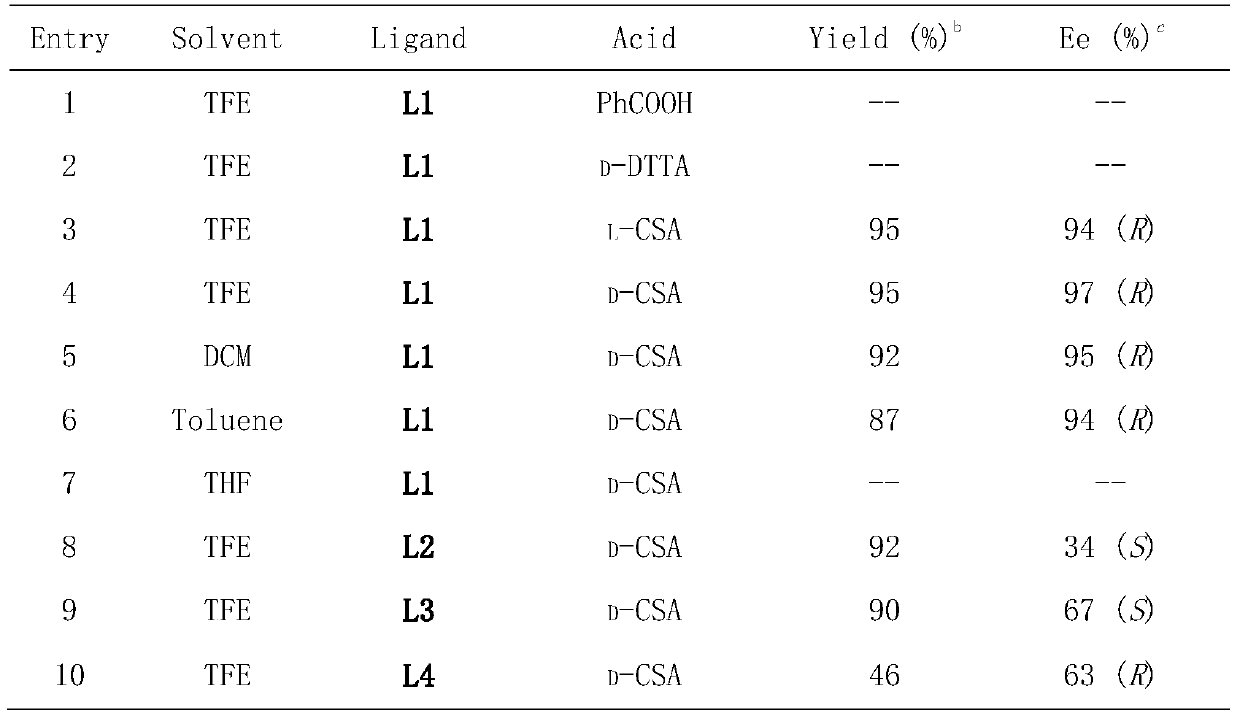
[0032] Drop into palladium trifluoroacetate (3mol% of substrate consumption in formula 1) and chiral phosphine ligand (S, S)-f-binaphane (3.3mol% of substrate consumption in formula 1) in reaction flask, nitrogen displacement Then 1 ml of acetone was added and stirred at room temperature for 1 hour. Concentrate in vacuo then, add 3 milliliters of trifluoroethanol under nitrogen, this solution is transferred to the reaction kettle that has put substrate 1a (51.8 mg, 0.2 mmol) and camphorsulfonic acid (100mol% of substrate consumption in formula 1) in advance , 600 psi of hydrogen was passed through, and the reaction was carried out at 50° C. for 24 hours. Slowly release hydrogen, remove the solvent, and directly separate the pure product by column chromatography. The reaction formula and ligand structure are as follows:
[0033]
[0034] The yield is the separation yield, and the enantiomeric excess of the product is determ...
Embodiment 2
[0038] Example 2: Synthesis of various chiral sulfonamides by palladium-catalyzed asymmetric reductive amination 2
[0039] Put palladium trifluoroacetate (3 mol% of the amount of substrate in formula 1) and (S, S,)-f-Binaphane (3.3 mol% of the amount of substrate in formula 1) in the reaction bottle, add 1 ml after nitrogen replacement Acetone, stirred at room temperature for 1 hour. Concentrate in vacuo then, add 3 milliliters of trifluoroethanol under nitrogen, this solution is transferred to the reaction kettle that has substrate (0.2 mmol) and D-CSA (100mol% of substrate consumption in formula 1) in advance, feeds Hydrogen to 600psi, react at 50°C for 15-24 hours, release hydrogen slowly. After removing solvent, direct column chromatography separates and obtains pure product, and reaction formula is as follows:
[0040]
[0041] The yield is the separation yield, and the enantiomeric excess of the product is determined by chiral liquid chromatography, see Table 2.
...
Embodiment 3
[0046] Example 3: Synthesis of various chiral sulfonamides by palladium-catalyzed asymmetric reductive amination
[0047] Put palladium trifluoroacetate (3 mol% of the amount of substrate in formula 1) and (S, S,)-f-Binaphane (3.3 mol% of the amount of substrate in formula 1) in the reaction bottle, add 1 ml after nitrogen replacement Acetone, stirred at room temperature for 1 hour. Concentrate in vacuo then, add 3 milliliters of trifluoroethanol under nitrogen, this solution is transferred to the reaction kettle that has substrate (0.2 mmol) and D-CSA (100mol% of substrate consumption in formula 1) in advance, feeds Hydrogen to 600psi, react at 50°C for 15-24 hours, release hydrogen slowly. After removing solvent, direct column chromatography separates and obtains pure product, and reaction formula is as follows:
[0048]
[0049] The yield is the isolated yield, and the enantiomeric excess of the product is determined by chiral liquid chromatography, see Table 3.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com