Preparation method and application of tilapia mossambica-derived streptococcus agalactiae recombinant CBP protein vaccine
A technology of streptococcus lactis and protein vaccines, applied in the field of molecular vaccinology, can solve the problems of ineffective Streptococcus iniae and achieve the effects of simple preparation method, low expression cost and safe preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
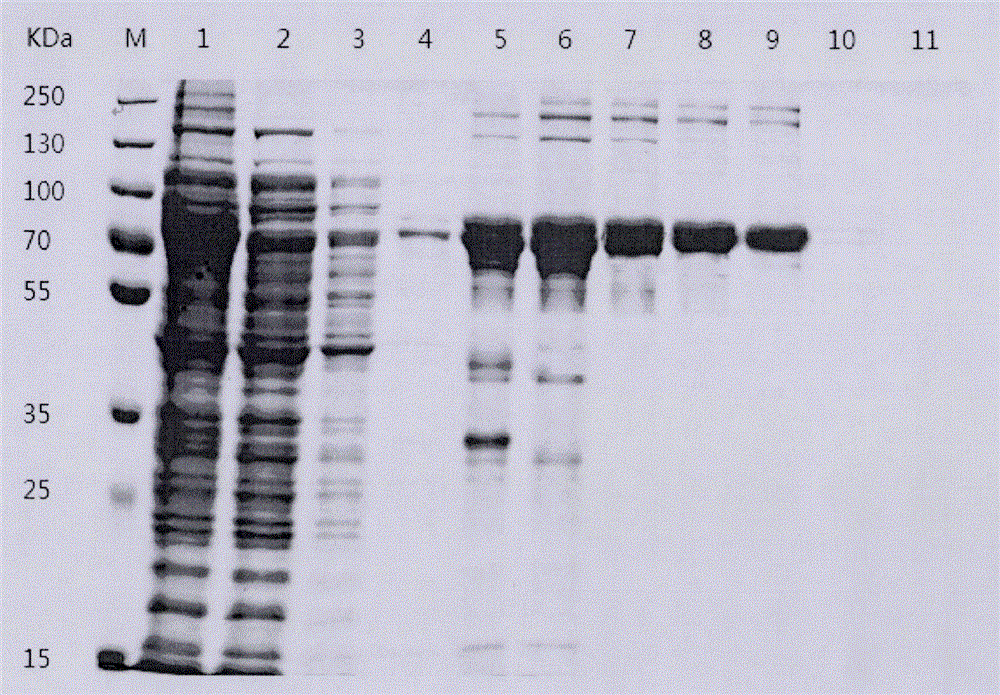
Image
Examples
Embodiment 1
[0050] Example 1 Preparation of tilapia-derived Streptococcus agalactiae recombinant CBP protein
[0051] 1. Primer design:
[0052] Using tilapia-derived Streptococcus agalactiae as a template, a pair of primers CBP-F and CBP-R containing restriction enzymes were designed, the sequences are as follows:
[0053] Primer CBP-F (sequence shown in sequence 3):
[0054] 5'- GGGGGGTCTCTAGTG GATCAAACTACATCGGTTCAA-3’
[0055] Primer CBP-R ((sequence is shown in sequence 4)):
[0056] 5'- GCCGGGTCTCGTGGG AATTTCAATATAGCGACGAAT-3’
[0058] (1) PCR amplification of Cbp gene
[0059] The genomic DNA of Streptococcus agalactiae from tilapia was extracted, and the above-mentioned primers were used for PCR reaction of CBP-F and CBP-R with the genomic DNA as the template.
[0060] The PCR reaction system was: 25μl reaction system containing 50ng template DNA (genomic DNA), 2.5μl 10×PCRBuffer (Mg+), 2μl dNTP Mixture (2.5mM), 1μl CBP-F, 1μl CBP-R, 0.125μl r...
Embodiment 2
[0088] Example 2 Preparation of tilapia-derived Streptococcus agalactiae recombinant CBP protein vaccine
[0089] The purified recombinant CBP protein obtained in Example 1 was diluted to 1 mg / ml with sterilized PBS, and mixed with Freund's complete and incomplete adjuvant at 1:1 to prepare a subunit vaccine.
[0090] Take 1ml of the prepared vaccine and centrifuge at 3000×g for 5min. If the vaccine has no stratification, it can be used for subsequent immunization.
Embodiment 3
[0091] Example 3 Tilapia-derived Streptococcus agalactiae recombinant CBP protein vaccine immunization test
[0092] 1. Immune protection experiment
[0093] (1) Nile tilapia was purchased from Guangzhou Zhongxingou Aquaculture Development Co., Ltd., the size was 30±5.0g, and it was temporarily cultured for two weeks before the experiment.
[0094] (2) In the immunization experiment, tilapia were randomly divided into 3 groups, namely PBS group, PBS+adjuvant group and CBP vaccine group. There are 15 tails in each group, and three parallel groups are set up in each group.
[0095] The CBP group and the adjuvant group were injected intraperitoneally with 100 μl of recombinant antigen CBP and PBS mixed with an equal volume of Freund's adjuvant, and the PBS group was injected with 100 μl of sterile PBS.
[0096] 2. Immunization program
[0097] Tilapia were immunized twice, with Freund's complete adjuvant for the primary immunization and subunit vaccines for the booster immuniz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com