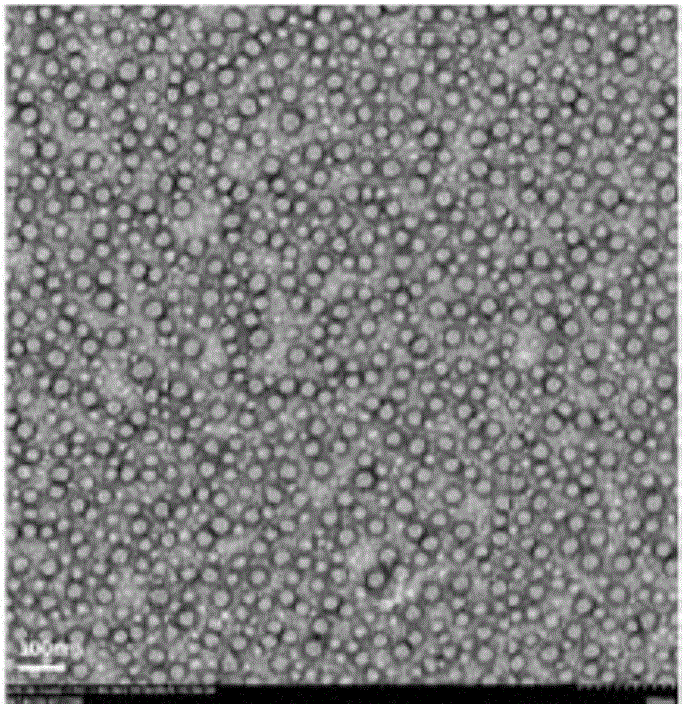
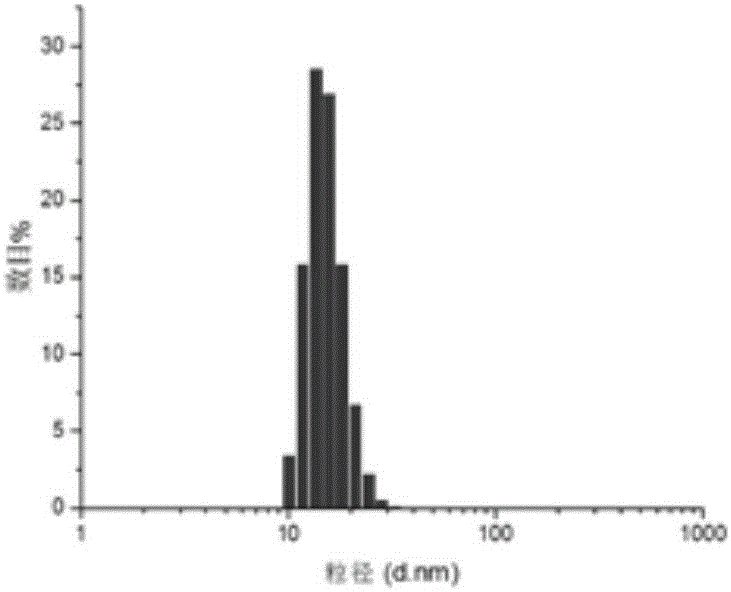
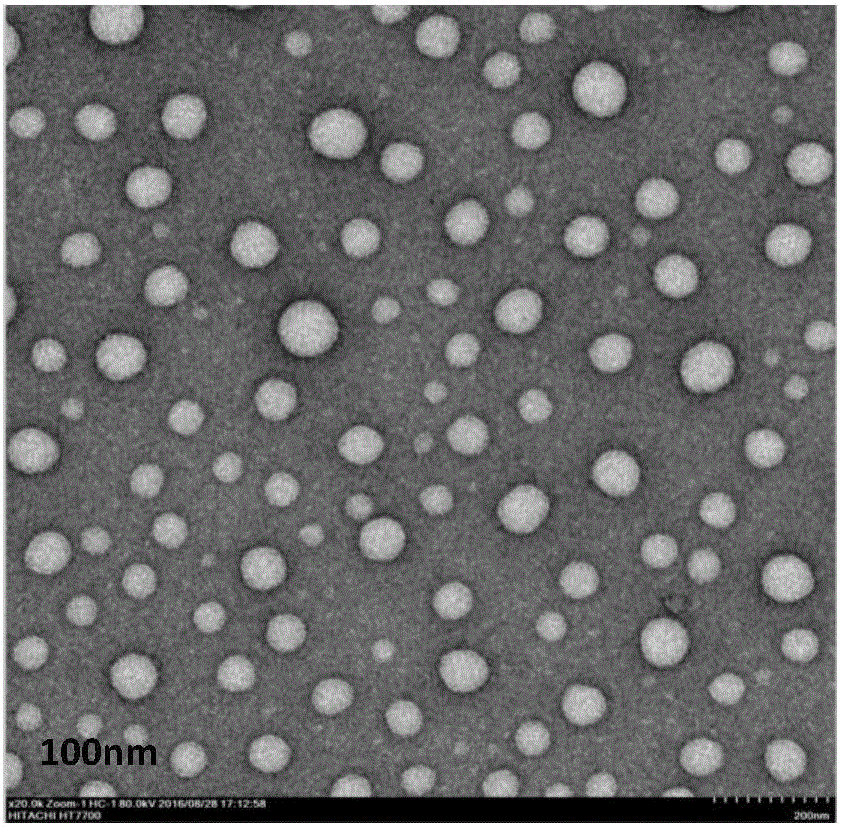
Specific target polypeptide self-assembled nano-carrier, drug-carrying nanoparticle and preparation method
A technology targeting peptides and nano-carriers, which can be used in pharmaceutical formulations, drug combinations, anti-tumor drugs, etc., can solve the problem of accumulation of toxicity, and achieve the effects of small side effects, good drug development potential, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] Wherein, the targeting peptide that can be recognized by the epidermal growth factor receptor at the tumor site is a peptide segment known in the art and can be purchased commercially. Alternatively, it can be prepared by Fmoc solid-phase synthesis known in the art. The present invention provides a specific preparation method. Those skilled in the art can understand that the method is not intended to limit the present invention. The raw materials involved in the following preparation method are commercially available, for example purchased from Jill Biochemical (Shanghai) Co., Ltd., Sigma-Aldrich Corporation.
[0043] (1) Take 1.01 g of dichlorotrityl chloride resin to the peptide synthesis device, add dry N,N-dimethylformamide to soak the resin for half an hour to make it fully swell, and finally discharge the solvent N,N-dimethylformamide base formamide.
[0044] (2) Weigh 0.2 g of Fmoc-Gly-OH and dissolve it with 5 ml of N,N-dimethylformamide, then transfer the sol...
Embodiment 1
[0049] A method for preparing a specific targeting polypeptide self-assembled nanocarrier, comprising the following steps:
[0050] (1) Preparation of amphiphilic polypeptide molecules: take 50 mg of the targeting peptide P-1 prepared by the above method, dissolve it in 5 mL of N,N-dimethylformamide solution, add 350 mg of octadecanoic acid, 2 mL of Catalyst diisopropylethylamine (DIEA), reacted at room temperature for 12 hours. After stopping the reaction, the liquid was added dropwise into anhydrous ether, and a white precipitate appeared immediately. Centrifuge (rotating speed: 5000rpm, centrifuge time: 5min) to separate the above suspension to remove the supernatant, freeze-dry the obtained product, and collect the white powder to obtain the amphiphilic polypeptide molecule.
[0051] (2) Preparation of nanocarriers: Dissolve amphiphilic polypeptide molecules in 20 uL of dimethyl sulfoxide solution at room temperature, disperse them in 1 mL of aqueous solution under ultras...
Embodiment 2
[0053] A method for preparing specific targeting polypeptide self-assembled drug-loaded nanoparticles, comprising the following steps:
[0054] (1) Preparation of amphiphilic polypeptide molecules: take 50 mg of the targeting peptide P-1 prepared by the above method, dissolve it in 5 mL of N,N-dimethylformamide solution, add 350 mg of octadecanoic acid, 2 mL of Catalyst diisopropylethylamine (DIEA), reacted at room temperature for 12 hours. After stopping the reaction, the liquid was added dropwise into anhydrous ether, and a white precipitate appeared immediately. Centrifuge (5000 rpm, centrifugation time 5 min) to separate the above suspension to remove the supernatant, freeze-dry the obtained product, and collect the white powder to obtain the amphiphilic polypeptide molecule, which is named P-2.
[0055] (2) Preparation of drug-loaded nanoparticles: at room temperature, according to the molar ratio of the drug to the amphiphilic polypeptide molecule being 1: (5-20), 1 mg ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com