Terminal hydroxyl organic nitrogen-sulfur-contained ester, preparation method thereof and flame-retardant Dacron manufactured by the same
A flame-retardant polyester and hydroxyl-terminated technology, which is applied in the field of flame-retardant polyester fabrics, can solve the problems of environmental pollution during the preparation process, difficulties in storage and use, and the content of small functional elements, and achieve the goal of overcoming easy migration, environmental protection, and good synergy The effect of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A preparation method of hydroxyl-terminated organic nitrogen-containing thioester compound, the steps are as follows:
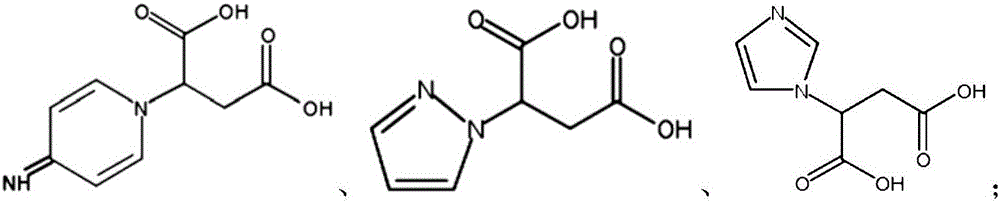
[0048] 1) Mix side group nitrogen-containing dicarboxylic acid and pentaerythritol in a molar ratio of 4:1, carry out melt esterification reaction under argon protection and mechanical stirring, the stirring speed of mechanical stirring is 300rpm, melt esterification reaction The temperature is 180°C, and the reaction time is 1h. After the reaction, the product is collected, dissolved, filtered and dried to obtain DAPER whose end group is carboxyl group. The structural formula of side group nitrogen-containing dicarboxylic acid is:
[0049]
[0050] 2) Mix organic nitrogen-sulfur dicarboxylic acid and ethylene glycol at a molar ratio of 1:2, add 4-methylbenzenesulfonic acid, organic nitrogen-sulfur dicarboxylic acid and 4-methylbenzenesulfonic acid The molar ratio of the acid is 1:0.01, the melt condensation reaction is carried out under the conditi...
Embodiment 2
[0062] A preparation method of hydroxyl-terminated organic nitrogen-containing thioester compound, the steps are as follows:
[0063] 1) Mix side group nitrogen-containing dicarboxylic acid and pentaerythritol in a molar ratio of 4:1, carry out melt esterification reaction under argon protection and mechanical stirring, the stirring speed of mechanical stirring is 350rpm, melt esterification reaction The temperature is 185°C, and the reaction time is 1h. After the reaction, the product is collected, dissolved, filtered and dried to obtain DAPER whose end group is carboxyl group. The structural formula of side group nitrogen-containing dicarboxylic acid is:
[0064]
[0065] 2) Mix the organic nitrogen-sulfur dicarboxylic acid and propylene glycol at a molar ratio of 1:2, add 4-methylbenzenesulfonic acid, the mixture of organic nitrogen-sulfur dicarboxylic acid and 4-methylbenzenesulfonic acid The molar ratio is 1:0.01, the melt condensation reaction is carried out under the...
Embodiment 3
[0077] A preparation method of hydroxyl-terminated organic nitrogen-containing thioester compound, the steps are as follows:
[0078] 1) Mix side group nitrogen-containing dicarboxylic acid and pentaerythritol in a molar ratio of 4:1, carry out melt esterification reaction under argon protection and mechanical stirring, the stirring speed of mechanical stirring is 400rpm, melt esterification reaction The temperature is 190°C, and the reaction time is 2 hours. After the reaction, the product is collected, dissolved, filtered and dried to obtain DAPER with a carboxyl group at the end. The structural formula of the side nitrogen-containing dicarboxylic acid is:
[0079]
[0080] 2) Mix organic nitrogen-sulfur dicarboxylic acid and 1,4-butanediol at a molar ratio of 1:2, add 4-methylbenzenesulfonic acid, organic nitrogen-sulfur dicarboxylic acid and 4- The molar ratio of toluenesulfonic acid is 1:0.01, the melt condensation reaction is carried out under the conditions of nitrog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specification | aaaaa | aaaaa |
| Limiting oxygen index | aaaaa | aaaaa |
| Limiting oxygen index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com