Fluorescent probe PMPA as well as preparation method and application of fluorescent probe PMPA
A fluorescent probe and fluorescence ratio technology, applied in the field of fluorescent probes, can solve the problem of less fluorescent probes, and achieve the effects of low detection limit, high sensitivity and simple synthesis steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The preparation of embodiment 1 fluorescent probe
[0052] (1) Add 0.7436g (3.0mmol) 1-acetylpyrene, 0.5482g (4.0mmol) 6-methoxy-pyridine-dicarbaldehyde and 60mL absolute ethanol to a 250mL two-necked flask respectively, at room temperature (20°C) Stir magnetically for 10 minutes until the reactant is completely dissolved and becomes a light yellow solution;
[0053] (2) Then add 3mL of 10% sodium hydroxide solution, the reaction solution turns yellow, and continue stirring at normal temperature for 32 hours, TLC tracking reaction (V 乙酸乙酯 :V 石油醚 =1:10);
[0054] (3) During the period, the yellow solution gradually turned into yellow crystals and stuck on the wall of the flask. After adding absolute ethanol, a large amount of yellow flocculent precipitates were generated; left standing, suction filtered, and dried to obtain 0.5211g of yellow solid product, with a yield of 47.1%. .
[0055] For fluorescent probes 1 H NMR characterization, the results are as follows: ...
Embodiment 2
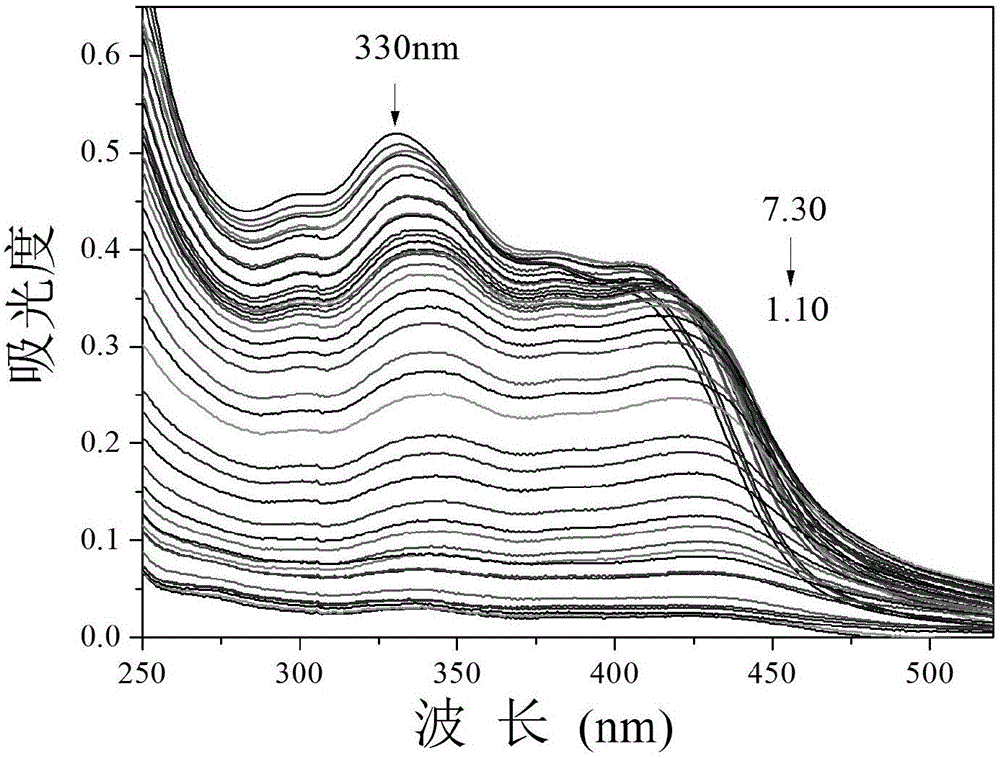
[0058] Embodiment 2 Fluorescence probe changes with the ultraviolet absorption spectrogram of acidity
[0059] Add 30.0 μL fluorescent probe stock solution to 2.0 mL distilled water system, and adjust the pH value with HCl, and record its ultraviolet absorption spectrum ( figure 1 ). With the weakening of acidity, the UV absorption at 330nm decreases gradually.
Embodiment 3
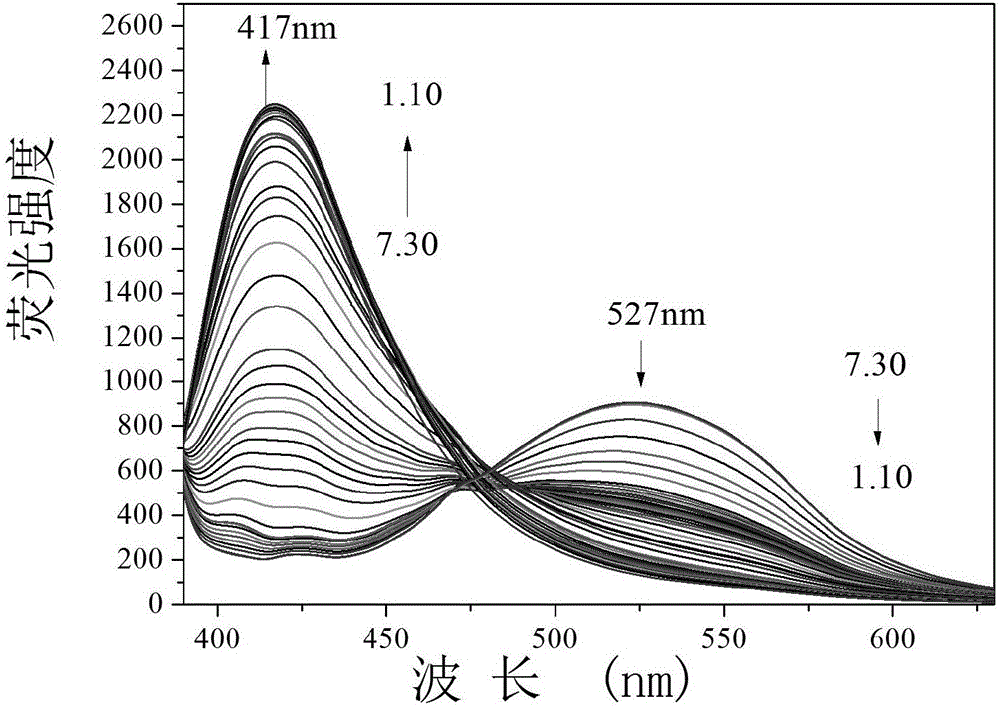
[0060] Fluorescence titration figure of embodiment 3 fluorescent probe PMPA changing with acidity
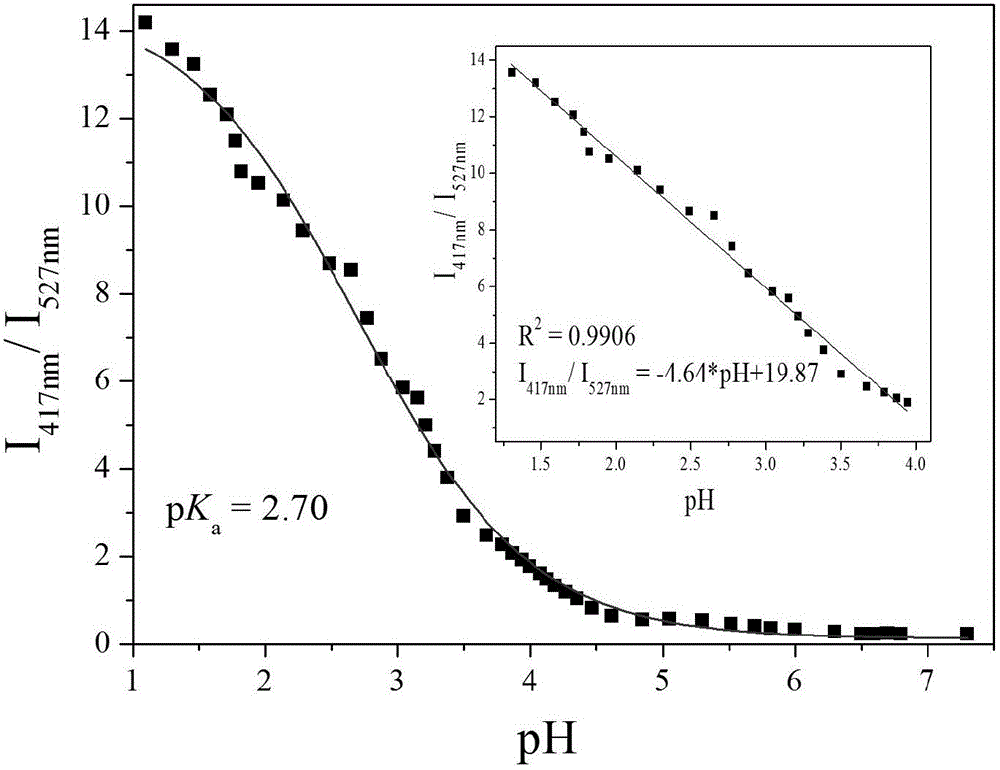
[0061] Add 30.0 μL of fluorescent probe stock solution to 2.0 mL of distilled water, adjust the pH value of the system with HCl, and detect it on a fluorescence spectrophotometer. With the increase of acidity, the fluorescence intensity at 527 nm gradually weakens, and the fluorescence at 417 nm The strength gradually increases ( figure 2 ). Instrument parameters: the slit widths of the excitation wavelength and emission wavelength are 5.0nm and 5.0nm respectively, the voltage is 600V, and the maximum excitation wavelength of the fluorescent probe solution is: λ ex is 370nm and the maximum emission wavelength is λ em 527nm. Relative fluorescence intensity I 417nm / I 527nm To plot the pH value and use Sigmoidal for fitting to obtain pK a =2.70; It is pH 1.26-3.97 to obtain the best linear response range of PMPA by linear fitting, and regression equation is: I 417nm / I 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com