Fast qualitative and quantitative detection method of oil adjuvant vaccine
A quantitative detection method and quantitative detection technology, applied in the field of foot-and-mouth disease synthetic peptide vaccine detection, can solve the problems of not responding well to the effective antigen content, and there is no universal and accurate method for quantitative detection, so as to achieve short time consumption, improve detection sensitivity, The effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] This embodiment provides a rapid qualitative and quantitative detection method for oil adjuvant vaccines, specifically using the following steps:
[0045] 1) The commercially available porcine foot-and-mouth disease synthetic peptide vaccine (vaccine antigen concentration is 50 μ g / mL) is demulsified according to the following method:
[0046] Take 10ml of the vaccine to be tested and mix it with n-butanol at a volume ratio of 1:1, add 50mg of histidine, shake and mix well, and centrifuge at 3000r / min for 15 minutes at 4°C, and carefully extract the lower layer of water with a 10ml syringe after centrifugation Phase, that is, the aqueous phase antigen sample.
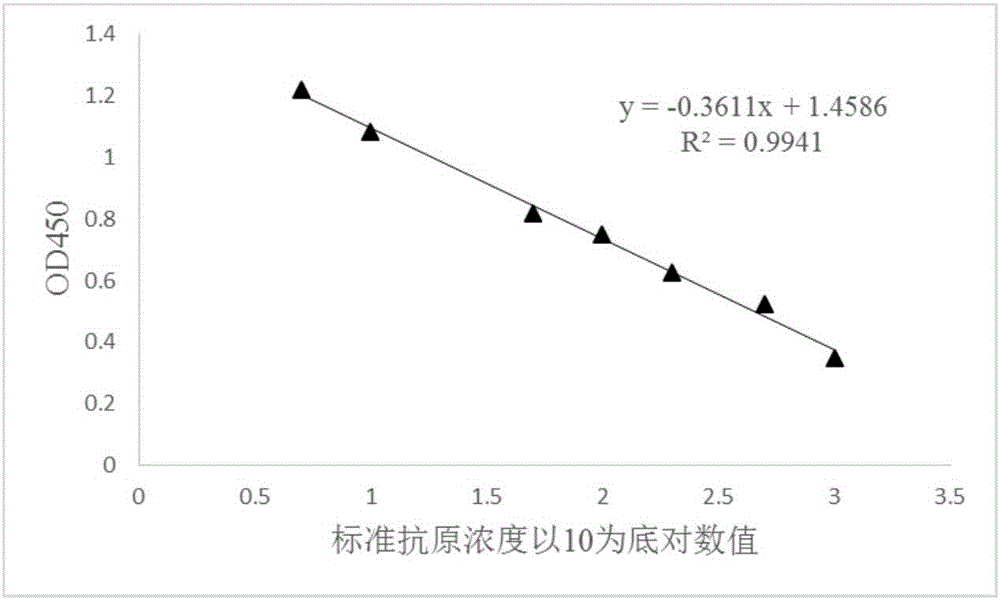
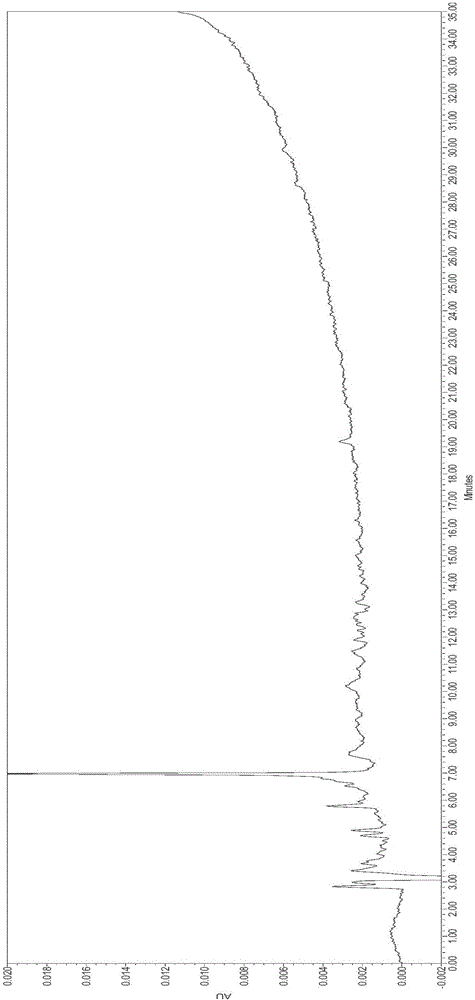
[0047] The commercially available pig foot-and-mouth disease synthetic peptide vaccine used in this embodiment is compared with the theoretical antigen concentration standard, and the peak position of its HPLC detection chromatogram sample is integrated, and its integral information is shown in Table 1. In the f...
Embodiment 2
[0064] This embodiment provides a rapid qualitative and quantitative detection method for oil adjuvant vaccines, specifically using the following steps:
[0065] 1) The commercially available porcine foot-and-mouth disease synthetic peptide vaccine (vaccine antigen concentration is 50 μ g / mL) is demulsified according to the following method:
[0066] Take 10ml of the vaccine to be tested and mix it with n-butanol at a volume ratio of 1:1, add 10mg of phenylalanine, shake and mix well, centrifuge at 3000r / min for 15 minutes at 4°C, and carefully extract the lower layer with a 10ml syringe after centrifugation Aqueous phase, namely the aqueous phase antigen sample.
[0067] The foot-and-mouth disease vaccine was demulsified by the method of this example, and the demulsification efficiency was 88.4%.
[0068] 2) Competitive ELISA detection
[0069] 2.1 Antigen coating: 3 μg / mL artificially synthesized FMD antigen, 100 μL per well, was immobilized onto the ELISA plate;
[0070]...
Embodiment 3
[0082] This embodiment provides a rapid qualitative and quantitative detection method for oil adjuvant vaccines, specifically using the following steps:
[0083] 1) The commercially available porcine foot-and-mouth disease synthetic peptide vaccine (vaccine antigen concentration is 50 μ g / mL) described in embodiment 1 is demulsified according to the following method:
[0084] Take 10ml of the vaccine to be tested and mix it with n-butanol at a volume ratio of 1:1, add 200mg of proline to each tube, vortex and mix well, centrifuge at 3000r / min for 15 minutes at 4°C, and carefully extract with a 10ml syringe after centrifugation The lower aqueous phase is the aqueous phase antigen sample.
[0085] The foot-and-mouth disease vaccine was demulsified by the method of this example, and the demulsification efficiency was 95.6%.
[0086] 2) Competitive ELISA detection
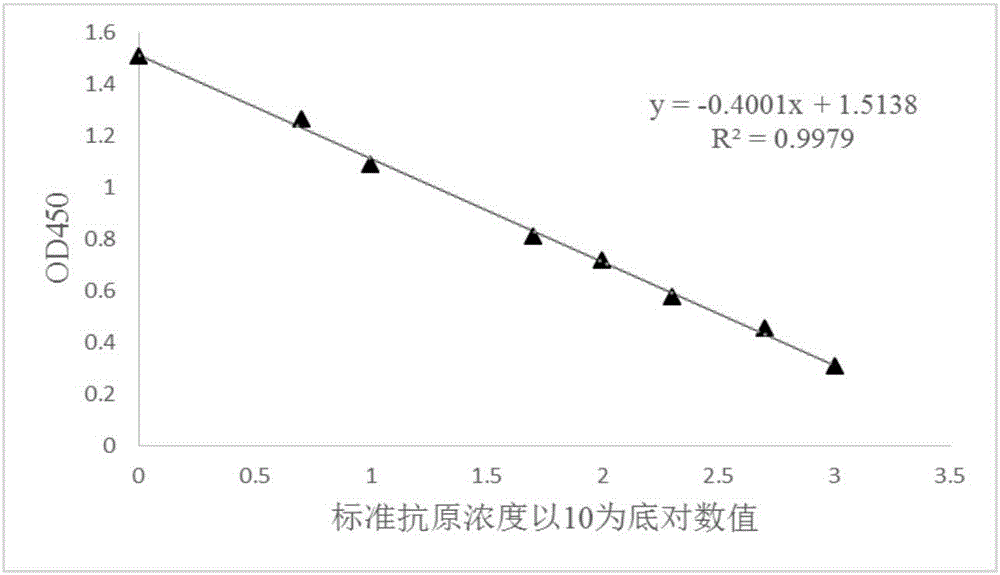
[0087] The competition ELISA method used was the same as in Example 2. The actual content of the antigen in the s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| demulsification efficiency | aaaaa | aaaaa |
| demulsification efficiency | aaaaa | aaaaa |
| demulsification efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com