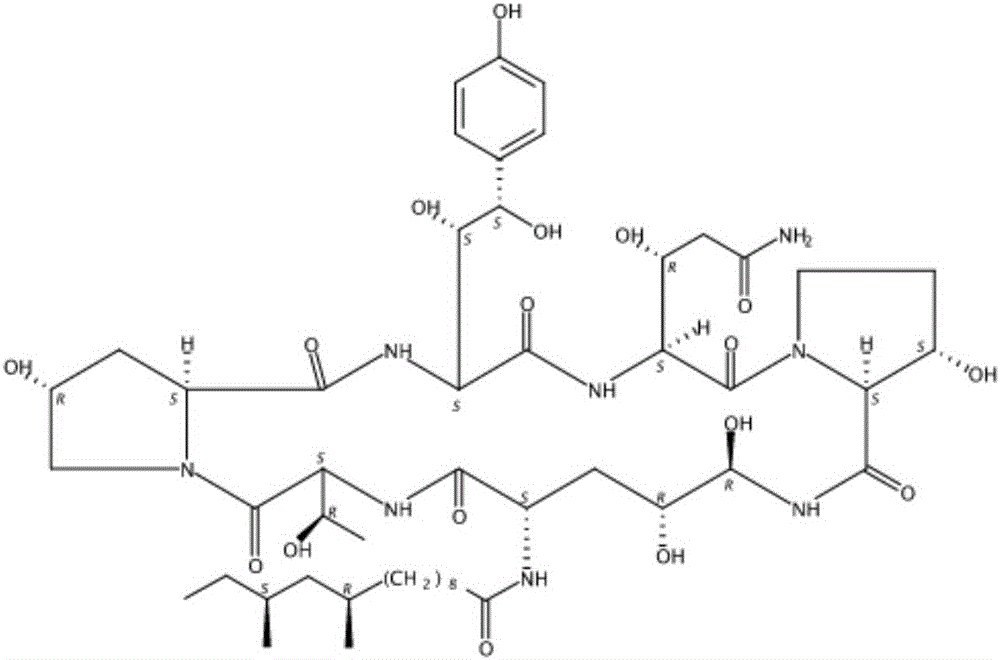
Fermentation method of caspofungin fermentation intermediate
A fermentation method and fermentation process technology, applied in the field of bioengineering, can solve the problems affecting the quality of caspofungin acetate, etc., and achieve the effect of improving the ventilation effect and reducing the viscosity of the bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Fermentation strain: Glarea lozoyensis
[0025] Fermentation medium (wt): lactose 3.0%, threonine 1.0%, yeast powder 1.0%, proline 1.2%, KH 2 PO 4 0.15%, magnesium sulfate heptahydrate 0.05%, MES buffer salt 1.5%, pH 5.3.
[0026] 50L fermenter culture, 30L medium, sterilized at 121°C for 30 minutes. The seed volume is 1.5L, the culture temperature of the fermentation broth is 25°C, the initial ventilation volume is 0.9VVM, 200 rpm, and the tank pressure is 0.05Mpa. The maximum amount is 1.2VVM. After 24 hours of fermentation, ammonia water was added to control the pH to 5.0-5.4. After 48 hours of fermentation, start to add vitamin b5, the added amount is 30mg / l. After 60 hours of fermentation, start to feed lactose, the flow rate is 2.0% / day (mass volume ratio), so that the total sugar concentration of the fermentation broth is controlled between 1-3%, until the end of fermentation. After 72 hours of fermentation (at this time, the ventilation rate and rotation s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com