Method for preparing high-purity sugammadex
A technology of high-purity sugammadex sodium, applied in the field of medicine, can solve the problems of safety, difficulty in preparing high-quality sugammadex sodium, and achieves improved quality and yield, significant industrial application value, purification Efficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
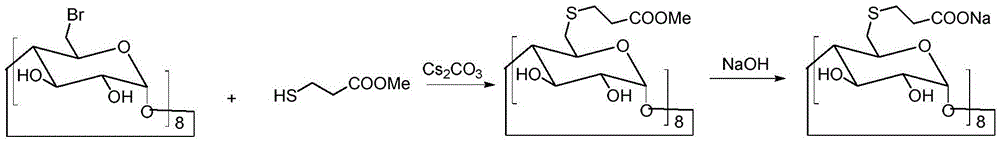
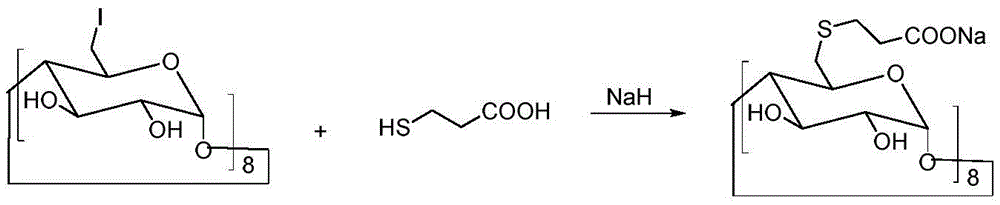
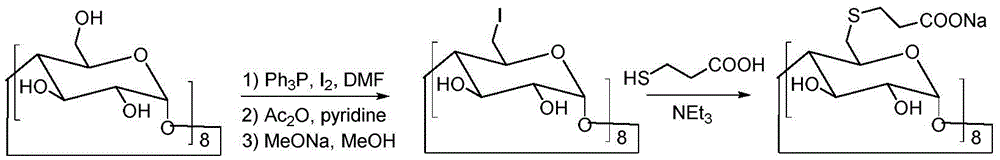
[0033] Under the conditions of ice bath and nitrogen protection, sodium hydroxide (4.4 g, 0.11 mol) was dispersed in 100 mL of N,N-dimethylformamide, and 3-mercaptopropionic acid (5.3 g, 0.05 mol) was slowly added dropwise, Keep the temperature at 0-5°C. After the dropwise addition was completed, the temperature was raised to room temperature, and the reaction was continued for 30 minutes. Cool down to 0°C again, slowly add 6-perdeoxy-6-periodo-γ-cyclodextrin (10.9 g, 5 mmol) in N,N-dimethylformamide solution dropwise, and raise the temperature to 80°C after the addition Reaction 20h. Concentrate the reactant under reduced pressure to a small volume, add 20 mL of water and 150 mL of ethanol to disperse, stir, and filter to obtain 8.3 g of a crude product with a yield of 76%.
Embodiment 2
[0035]
[0036] Under the conditions of ice bath and nitrogen protection, sodium hydroxide (4.4 g, 110 mmol) was dispersed in 100 mL of N,N-dimethylformamide, and 3-mercaptopropionic acid (5.3 g, 50 mmol) was slowly added dropwise to keep the temperature at 0-5°C. After the dropwise addition was completed, the temperature was raised to room temperature, and the reaction was continued for 30 minutes. Cool down to 0°C again, slowly add 6-perdeoxy-6-perbromo-γ-cyclodextrin (9 g, 5 mmol) in N,N-dimethylformamide solution dropwise, and raise the temperature to 80°C after the addition Reaction 20h. Concentrate the reactant under reduced pressure to a small volume, add 20 mL of water, and 150 mL of ethanol to disperse, stir, and filter to obtain 7.7 g of crude product, with a yield of 71%.
Embodiment 3
[0038]
[0039]Under the conditions of ice bath and nitrogen protection, sodium hydroxide (4.4 g, 110 mmol) was dispersed in 100 mL of N,N-dimethylformamide, and 3-mercaptopropionic acid (5.3 g, 50 mmol) was slowly added dropwise to keep the temperature at 0-5°C. After the dropwise addition was completed, the temperature was raised to room temperature, and the reaction was continued for 30 minutes. Cool down to 0°C again, slowly add 6-perdeoxy-6-perbromo-γ-cyclodextrin (7.2 g, 5 mmol) in N,N-dimethylformamide solution dropwise, and raise the temperature to 100°C after the addition Anti-24 hours. Concentrate the reactant under reduced pressure to a small volume, add 20 mL of water and 150 mL of ethanol to disperse, stir, and filter to obtain 6.7 g of a crude product with a yield of 62%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com