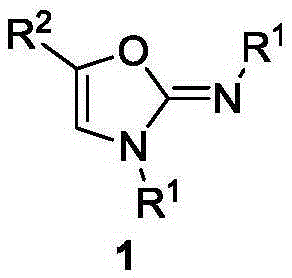
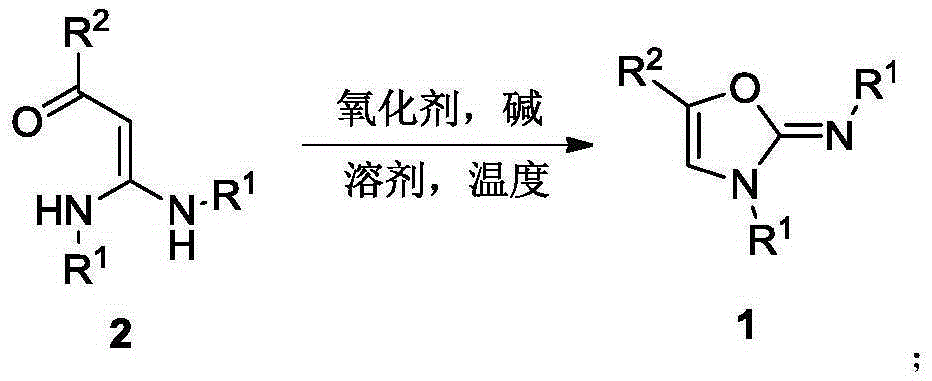
Method for synthesis of 2-imino oxazole
A technology of iminooxazole and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of few 2-iminooxazole synthesis methods, complicated substrate preparation, low reaction yield and the like, and achieve the target product yield High, cheap and easy to obtain raw materials, high product yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040] In a sealed 10 mL tube, add 1-phenyl-3,3-dianiline-2-propen-1-one 2a (94 mg, 0.3 mmol), iodophenyl acetate PhI(OAc) in sequence under air 2 (116mg, 0.36mmol), potassium carbonate K 2 CO 3 (83mg, 0.6mmol) and 2.5mL of dichloromethane were stirred at 80°C for 2 hours. After cooling to room temperature, filter through diatomaceous earth, remove volatile components under reduced pressure, and then separate by silica gel column chromatography (eluent is petroleum ether (60-90°C) / ethyl acetate / dichloromethane, v / v / v=15:1:1), the target product 1a (69 mg, yield 74%) was obtained, and the target product was confirmed by nuclear magnetic resonance spectroscopy.
Embodiment 2
[0042] Reaction steps and operation are with embodiment 1, and difference with embodiment 1 is that oxygenant is trifluoroacetic acid iodobenzene PhI (TFA) 2 . The reaction was stopped, and the target product 1a (9 mg, yield 10%) was obtained after post-processing. It shows that iodobenzene trifluoroacetate can also be used as the oxidizing agent for the reaction, but it is not the best oxidizing agent.
Embodiment 3
[0044]The reaction steps and operation are the same as in Example 1, except that the difference from Example 1 is that the oxidizing agent is potassium persulfate. The reaction was stopped, and the target product 1a (4 mg, yield 4%) was obtained after post-treatment. It shows that potassium persulfate is not conducive to the reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com