Cytomedicine for preventing intrauterine adhesion and repairing endometrium injury, and preparation method thereof
A cell preparation and endometrial technology, applied in the field of obstetrics and gynecology, can solve the problems of functional recovery of endometrial or short action time, inability to achieve endometrial regeneration and repair, inconvenient cell acquisition, etc., and achieve a lubricating biological barrier. Function, good degradability, growth-promoting and secretion-promoting effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Preparation of cell preparations for preventing intrauterine adhesions and repairing endometrial damage
[0053] The cell preparation for preventing intrauterine adhesions and repairing endometrial damage in this example is a suspension composed of third-generation umbilical cord or placental mesenchymal stem cells, rhSDF-1α and medical sodium hyaluronate gel. And the concentration of each component in the suspension is that the final concentration of umbilical cord or placental mesenchymal stem cells is 5×10 6 individual / mL, the mass concentration of rhSDF-1α was 10 ng / mL, the mass concentration of sodium hyaluronate gel was 10 mg / mL, and the molecular weight of sodium hyaluronate gel was 400,000.
[0054] Wherein, the above-mentioned umbilical cord mesenchymal stem cells are prepared by the following method:
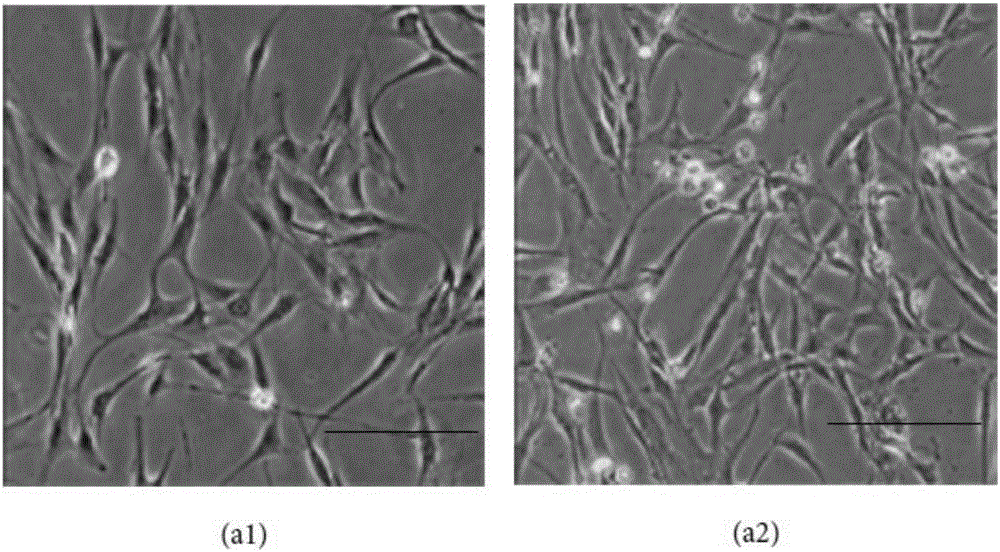
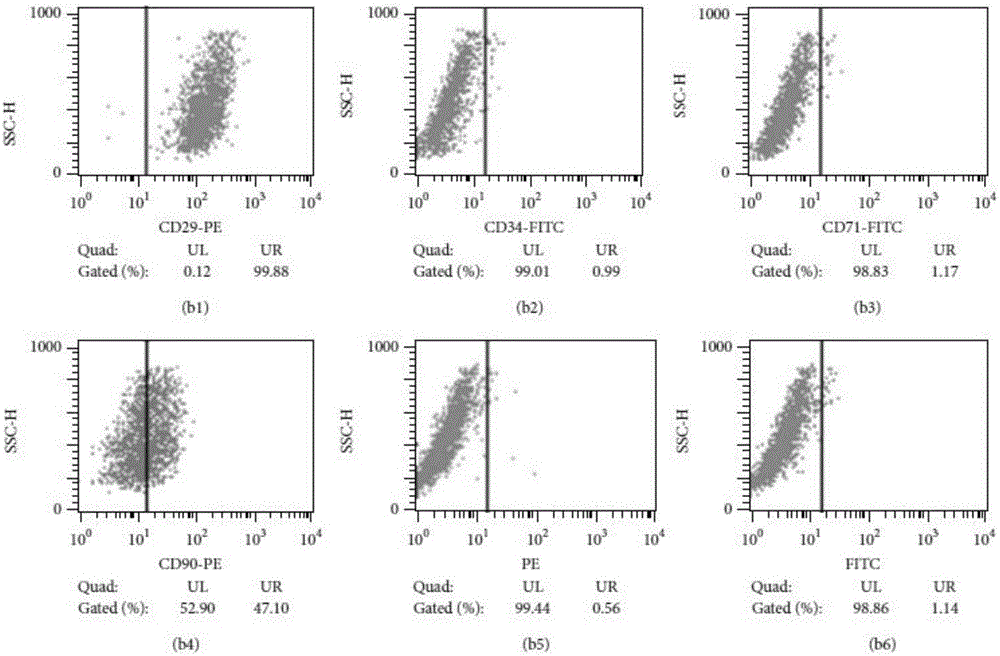
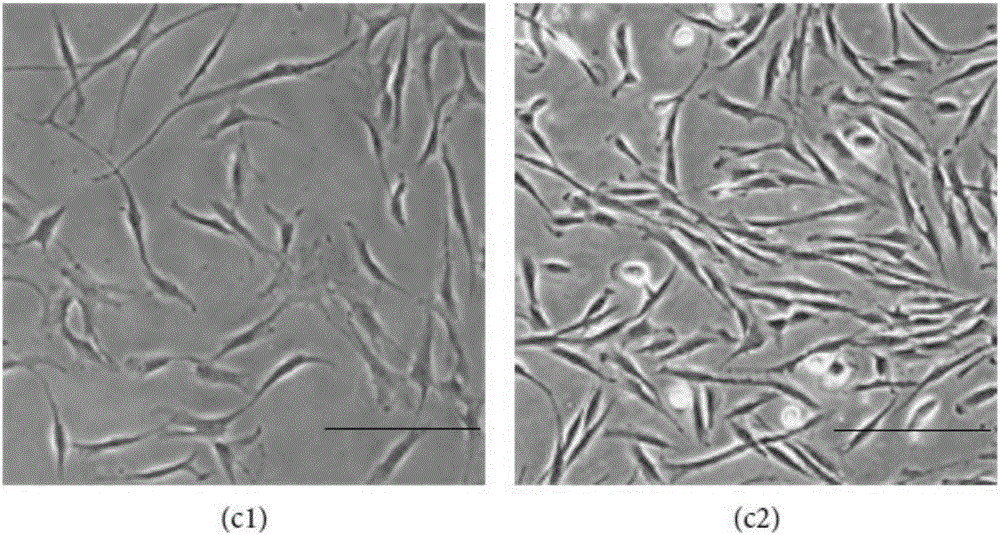
[0055] Take the umbilical cords of full-term cesarean section neonates from volunteers and healthy qualified pregnant women, wash them repeatedly wit...
Embodiment 2
[0058] Example 2 Preparation of cell preparations for preventing intrauterine adhesions and repairing endometrial damage
[0059] The cell preparation for preventing intrauterine adhesions and repairing endometrial damage in this example is a suspension composed of third-generation umbilical cord or placental mesenchymal stem cells, rhSDF-1α and medical sodium hyaluronate gel. And the concentration of each component in the suspension is the final concentration of umbilical cord or placental mesenchymal stem cells 1×10 7 per mL, the mass concentration of rhSDF-1α was 80 ng / mL, the mass concentration of sodium hyaluronate gel was 55 mg / mL, and the molecular weight of sodium hyaluronate gel was 700,000.
[0060] Wherein, the above-mentioned preparation method of umbilical cord mesenchymal stem cells is the same as that in Example 1.
[0061] Wherein, the above-mentioned preparation method of placental mesenchymal stem cells is the same as that in Example 1.
Embodiment 3
[0062] Example 3 Preparation of cell preparations for preventing intrauterine adhesions and repairing endometrial damage
[0063] The cell preparation for preventing intrauterine adhesions and repairing endometrial damage in this example is a suspension composed of third-generation umbilical cord or placental mesenchymal stem cells, rhSDF-1α and medical sodium hyaluronate gel. And the concentration of each component in the suspension is that the final concentration of umbilical cord or placental mesenchymal stem cells is 2×10 8 individual / mL, the mass concentration of rhSDF-1α was 150 ng / mL, the mass concentration of sodium hyaluronate gel was 100 mg / mL, and the molecular weight of sodium hyaluronate gel was 1,000,000.
[0064] Wherein, the above-mentioned preparation method of umbilical cord mesenchymal stem cells is the same as that in Example 1.
[0065] Wherein, the above-mentioned preparation method of placental mesenchymal stem cells is the same as that in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com