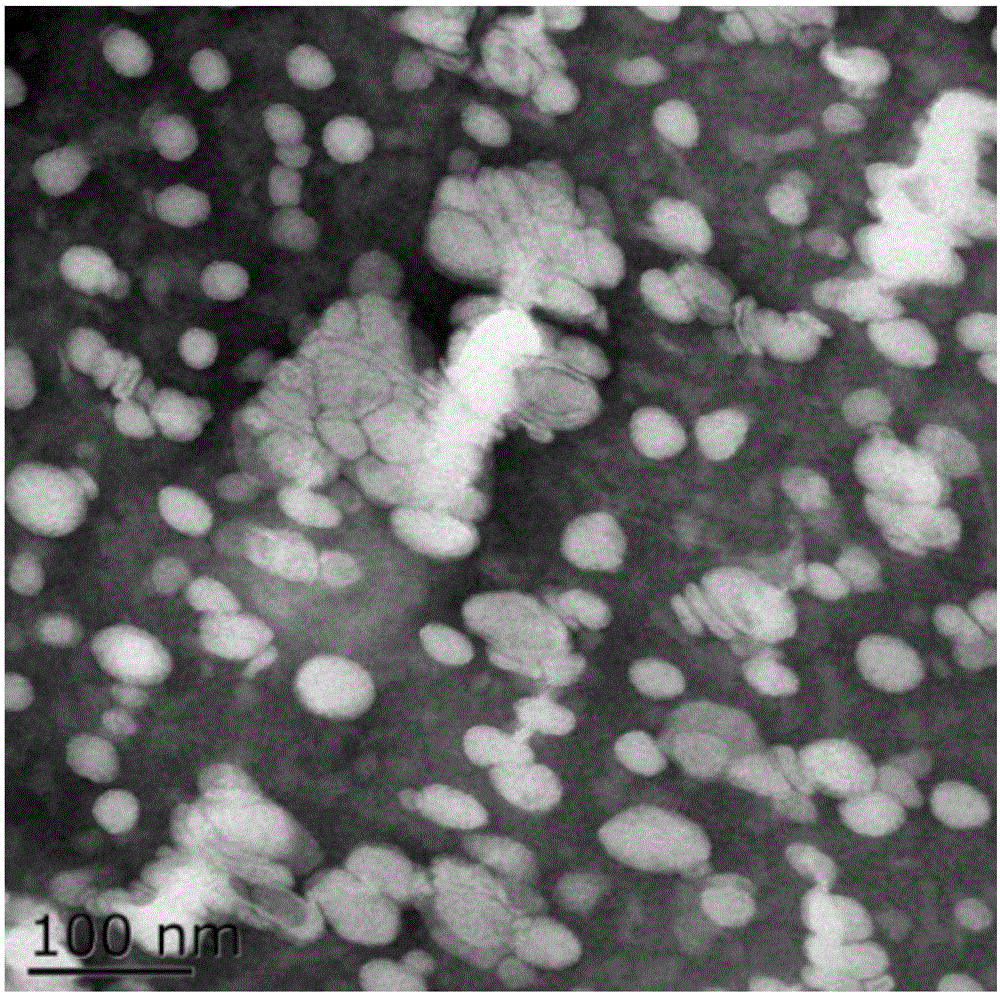
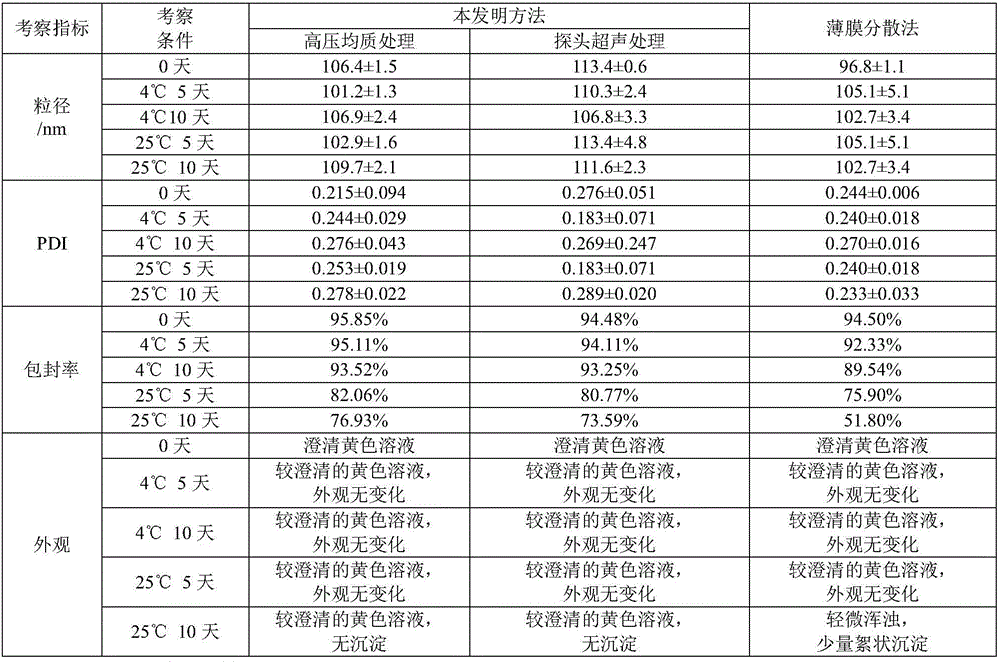
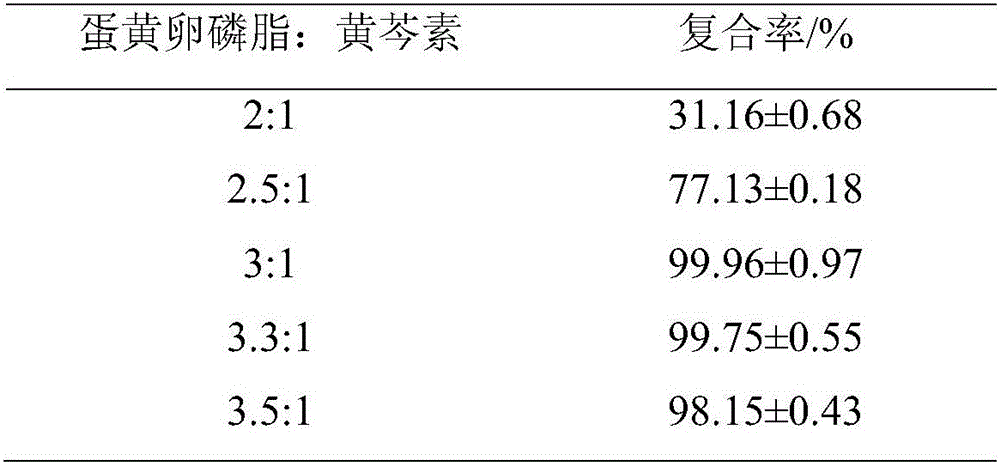
Liposome preparation method based on drug-phospholipid-cholesterol ternary complex
A technology of ternary complex and cholesterol, which is applied in the directions of liposome delivery, pharmaceutical formulations, medical preparations of inactive ingredients, etc., can solve the problems of unsuitability for industrialization, low production efficiency and high production cost, and achieves easy industrialization and amplification , High production efficiency, the effect of reducing the amount of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The liposome preparation method based on the drug-phospholipid-cholesterol ternary complex embodied by the present invention comprises:
[0033] The first step, the phospholipid of prescription amount is divided into the phospholipid of the first dosage and the phospholipid of the second dosage, and the first dosage is less than the second dosage; Get target medicine, the phospholipid of the first dosage and cholesterol put container, add reaction solvent to make material Dissolve completely, keep stirring at 30°C-80°C for 1-8h, then evaporate to remove the reaction solvent, place the product in vacuum for 8-16h, and then get powdery solid or spongy semi-solid Drug-phospholipid-cholesterol ternary complex; the quality of the first amount of phospholipid is at least 3 times that of the target drug, and the mass ratio of the target drug to cholesterol is 1:(0.1-10); 1-1.5 times of the minimum amount for complete dissolution, and the reaction solvent is one of tetrahydrofu...
Embodiment 1
[0041] The mass volume concentration (g / ml) of each component in the prescription is:
[0042] Baicalein 0.8%
[0044] Cholesterol 1.36%
[0045] The balance is pure water.
[0046] Preparation process: according to the above prescription, take baicalein, cholesterol and egg yolk lecithin with 3 times the quality of baicalein (that is, the mass ratio of baicalein to the first amount of egg yolk lecithin is 1:3, and the mass ratio of baicalein to cholesterol is 1:1.7), placed in an eggplant-shaped bottle, using tetrahydrofuran as a reaction solvent to completely dissolve the three, and after stirring at 30°C for 1 hour, evaporated to remove the solvent, and kept the product under vacuum for 12 hours to obtain baicalein-egg yolk lecithin- Cholesterol ternary complex, take out, avoid light, and store in a sealed place in a cool place. The essence of this step is to prepare the baicalein-egg yolk lecithin-cholesterol ternary complex by solvent met...
Embodiment 2
[0050] The mass volume concentration (g / ml) of each component in the prescription is:
[0051] Quercetin 0.2%
[0053] Cholesterol 0.4%
[0054] The balance is pure water.
[0055] Preparation process: according to the above prescription, weigh quercetin, cholesterol and egg yolk lecithin with 3.2 times the quality of quercetin (that is, the mass ratio of quercetin to the first amount of egg yolk lecithin is 1:3.2, quercetin and cholesterol The mass ratio is 1:2), placed in an eggplant-shaped bottle, using tetrahydrofuran as a reaction solvent to completely dissolve the three, and after stirring at 55°C for 4 hours, evaporated to remove the solvent, and kept the product under vacuum for 12 hours to obtain quercetin -Egg yolk lecithin-cholesterol ternary complex, take it out, keep it away from light, and keep it sealed in a cool place.
[0056] Weigh the remaining egg yolk lecithin in the prescription (i.e. the second dosage of egg yolk lecithin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com