Detection method of qualitative analysis of case drugs by utilizing micro-nuclear magnetic resonance spectrometer
A technology of nuclear magnetic resonance spectroscopy and detection method, which is applied in analysis by nuclear magnetic resonance, material analysis by resonance, preparation of samples for testing, etc. Low cost and small footprint
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 600MHz nuclear magnetic resonance spectrometer to detect two drug standard samples
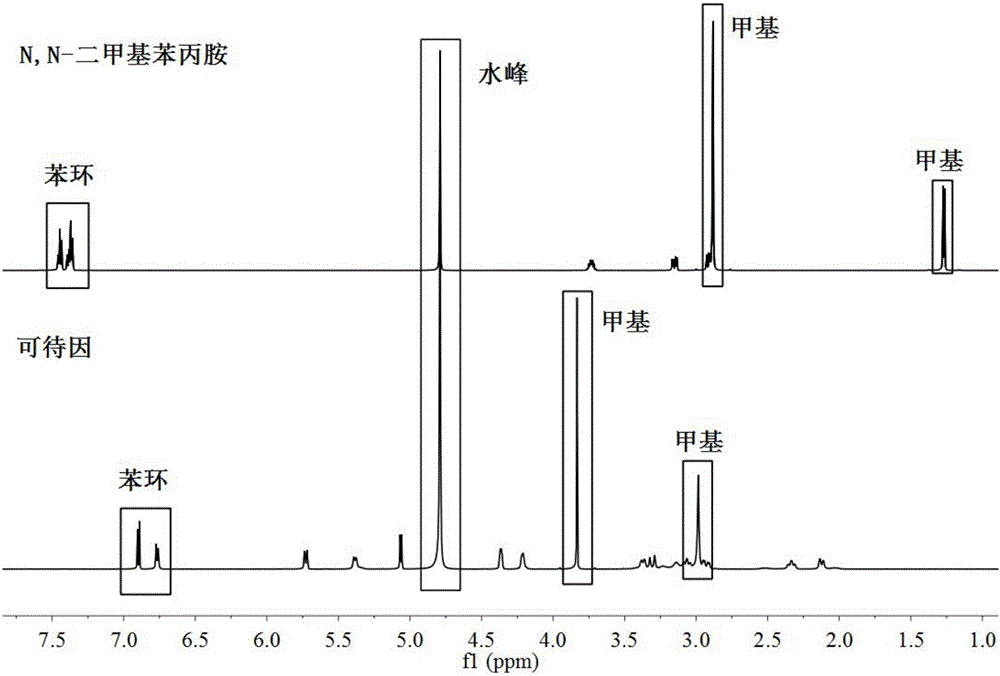
[0031] Weigh 10mg codeine and N,N-dimethylamphetamine standard samples respectively, put them in 2ml glass bottles, add 0.5mL deuterated water (containing 0.05% TMSP), transfer the standard samples to 5mm NMR tubes after complete dissolution . Put the 5mm nuclear magnetic tube containing the standard sample solution into the autosampler of the bruker 600MHz nuclear magnetic resonance spectrometer to carry out the hydrogen spectrum experiment, and the 600MHz hydrogen spectrum of the two drugs was obtained after the experiment. With reference to the chemical shift (0ppm) of internal standard TMSP, mark the 600MHz hydrogen spectrum chemical shift of these two kinds of drugs, obtain the 600MHz nuclear magnetic resonance spectrometer standard hydrogen spectrum of codeine and N,N-dimethylamphetamine, as figure 2 shown.
[0032] Depend on figure 2 It can be seen that the characteristic pe...
Embodiment 2
[0034] 80MHz miniature nuclear magnetic resonance spectrometer to detect three drug standard samples
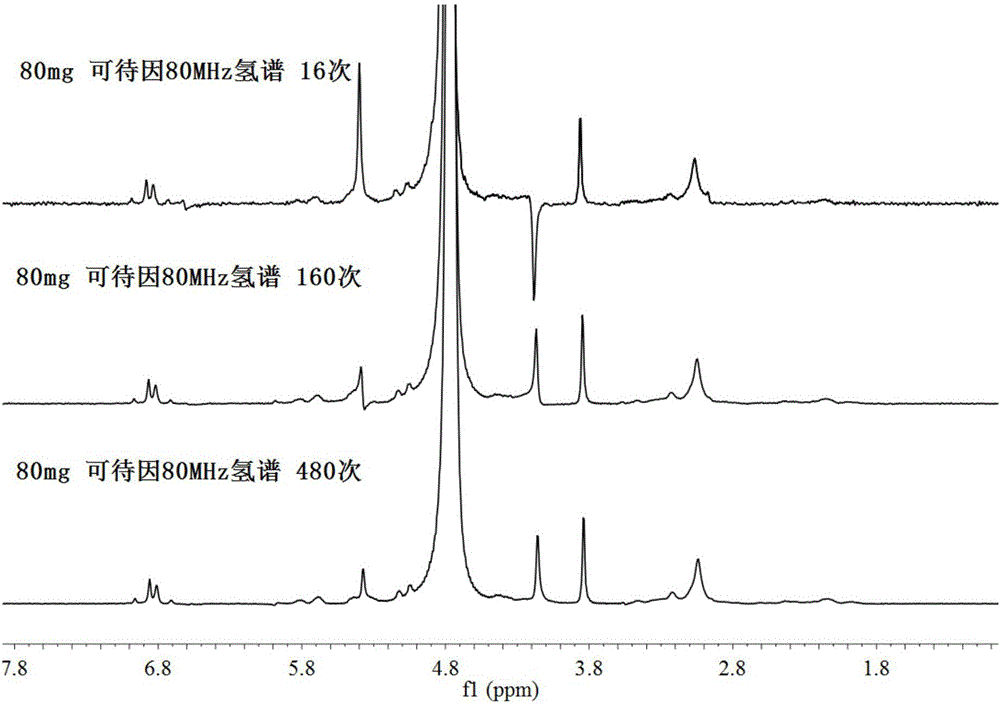
[0035] Weigh 50mg of morphine, 80mg of codeine and 50mg of N,N-dimethylamphetamine into three different 2ml glass bottles, add 1ml of deionized water, and dissolve them by ultrasonic. Use a 1ml syringe to draw the drug solution through a 0.45μm filter membrane and place it in a new 2ml glass bottle, then use a 1ml syringe to draw the filtrate and inject it into a picospin 80 miniature nuclear magnetic resonance spectrometer with an experimental temperature of 35°C, and select the onepulse mode for one-dimensional Hydrogen spectrum experiment, get 80MHz hydrogen spectrum of morphine, codeine and N,N-dimethylamphetamine, adjust the water peak chemical shift (4.79ppm) of morphine, codeine and N,N- The chemical shifts of the 80MHz hydrogen spectrum of dimethylamphetamine, while adjusting the baseline and characteristic peaks of the 80MHz hydrogen spectrum of morphine, codeine and...
Embodiment 3
[0041] With reference to the chemical shift (0ppm) of the internal standard TMSP in deuterated water, the 600MHz nuclear magnetic resonance spectrometer standard proton spectrum database of common drugs is established, as follows:
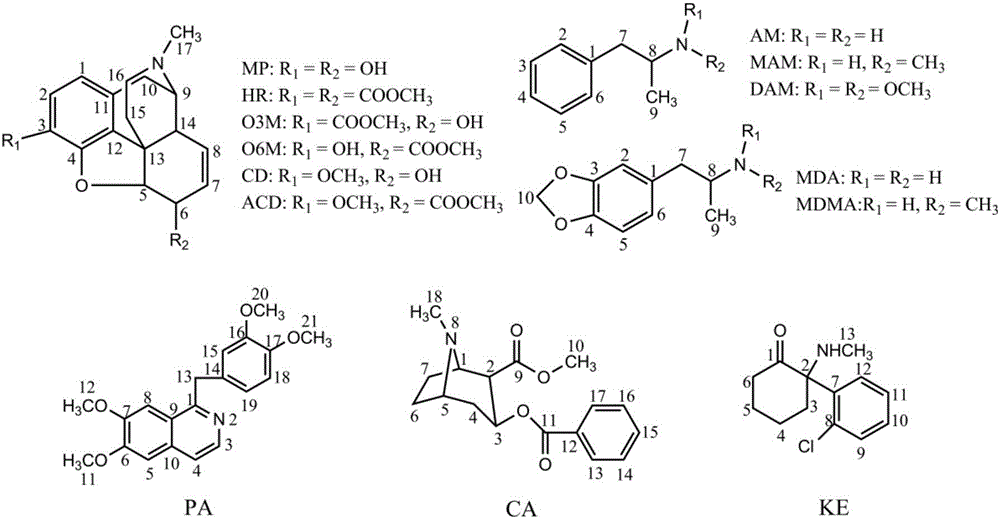
[0042] Weigh 10 mg of morphine (MP), O 3 - Monoacetylmorphine (O3M), O 6 - Monoacetylmorphine (O6M), codeine (CD), acetylcodeine (ACD), heroin (HR), papaverine (PA), cocaine (CA), amphetamine (AM), methamphetamine (MAM) , N,N-dimethylamphetamine (DAM), 3,4-methylenedioxyamphetamine (MDA), 3,4-methylenedioxymethamphetamine (MDMA) and ketamine, chemical structures such as figure 1 As shown, put in a 2ml glass bottle, add 0.5ml deuterated water (containing 0.05% TMSP), and transfer the drug standard sample to a 5mm glass NMR tube after completely dissolving, put the 5mm NMR tube containing the drug standard sample into the bruker One-dimensional and two-dimensional spectrogram experiments were performed in the autosampler of the 600MHz NMR spectrome...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com