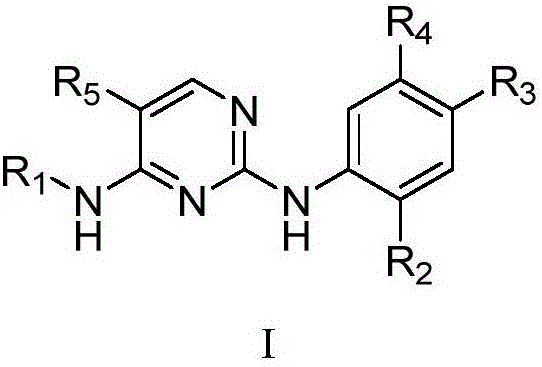
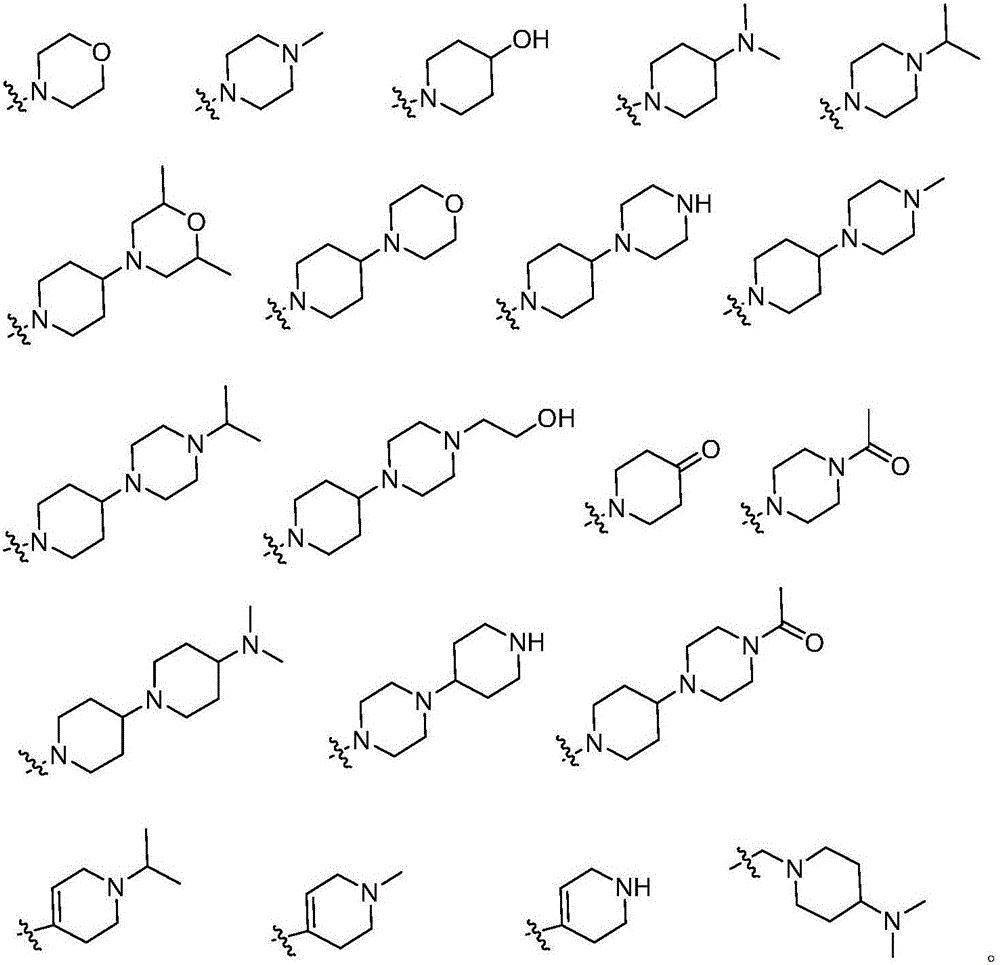
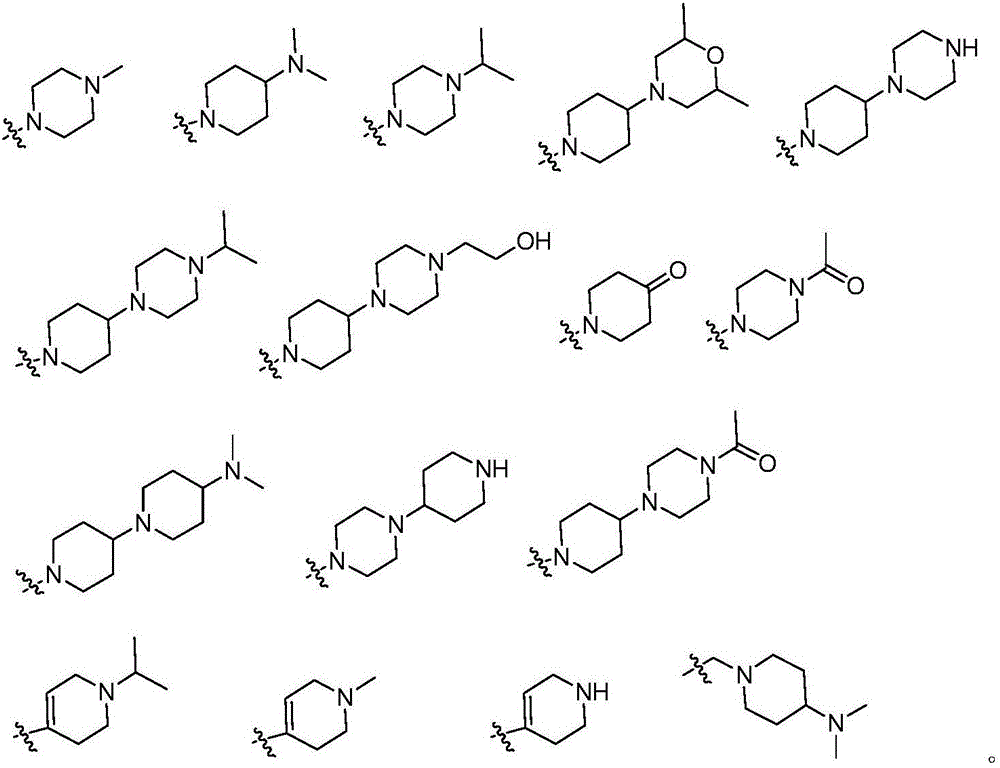
Pyrimidine derivative and application thereof
A technology of drugs and compounds, applied in the field of pyrimidine derivatives and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] The preparation process of compound shown in embodiment 1 formula I-17
[0126]
[0127] 1. Preparation of compounds shown in formula 3A-1
[0128] The compound shown in formula 1A-1 (0.5g, 2.92mmol), the compound shown in formula 2A-1 (0.5g, 2.52mmol) and potassium carbonate (807mg, 5.84mmol) were dissolved in anhydrous DMF (10mL), heated Raise the temperature to 80°C, stir the reaction for 5 hours, monitor the completion of the reaction by TLC, add 100 ml of ethyl acetate for extraction, wash with saturated brine three times, separate the liquids, dry the organic phase with anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain formula 3A The crude compound (0.8 g, yield 78.4%) shown in -1 was directly used in the next reaction without purification.
[0129] 2. Preparation of compounds shown in formula 4A-1
[0130] The compound shown in Formula 3A-1 (0.8g, 2.28mmol) was dissolved in THF / MeOH (v / v=1:1, 20ml in total) and 10ml of satura...
Embodiment 2
[0136] The preparation process of the compound shown in embodiment 2 formula I-12
[0137]
[0138] Dissolve the compound shown in Formula 4A-1 (41.85 mg, 0.131 mmol), the compound shown in Formula 5A-2 (45.48 mg, 0.131 mmol) in n-butanol (2 mL), and add p-toluenesulfonic acid (23 mg, 0.132mmol), heated to 100°C and stirred for 5 hours, TLC detection showed that the reaction was complete, concentrated under reduced pressure, and the resulting crude product was purified and separated by column chromatography to obtain an off-white solid product (35mg, yield 42.4%), which is the formula Compound shown in I-12.
[0139] 1 H NMR (400MHz, cd 3 od)δ9.11(d, J=8.6Hz, 1H), 8.34(dd, J=4.4, 1.3Hz, 1H), 8.10(s, 1H), 7.49(dd, J=12.8, 6.5Hz, 2H) ,6.68(d,J=2.4Hz,1H),6.56(dd,J=8.7,2.5Hz,1H),3.86–3.79(m,3H),3.79–3.72(m,2H),3.72–3.62(m ,2H),3.46(d,J=7.2Hz,1H),2.94(d,J=11.0Hz,2H),2.72(t,J=11.5Hz,2H),2.37(d,J=11.4Hz,2H ), 2.04(d, J=12.2Hz, 2H), 1.94(t, J=10.9Hz, 2H), 1.66(ddd, J=24.2, 12....
Embodiment 3
[0141] The preparation process of the compound shown in embodiment 3 formula I-4
[0142]
[0143] 1. Preparation of compounds shown in formula 3A-2
[0144] The compound shown in formula 1A-1 (0.5g, 2.92mmol), the compound shown in formula 2A-2 (254.5mg, 2.92.mmol) and potassium carbonate (1.21g, 8.77mmol) were dissolved in anhydrous DMF (10mL) , heated to 80°C, stirred and reacted for 5 hours, TLC monitored the completion of the reaction, added 100 ml of ethyl acetate for extraction, washed three times with saturated brine, separated liquids, dried the organic phase with anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain The crude compound represented by formula 3A-2 (566 mg, yield 81.3%) was directly used in the next reaction without purification.
[0145] 2. Preparation of compounds shown in formula 4A-1
[0146] The compound shown in Formula 3A-2 (0.54g, 2.28mmol) was dissolved in THF / MeOH (v / v=1:1, 20ml in total) and 10ml of satura...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com