Method for preparing 2-methylfuran
A technology of methyl furan and catalyst, applied in the field of preparation of 2-methyl furan, can solve the problems of low selectivity, environmental pollution, poor reusability, etc., achieve high activity and selectivity, convenient preparation process, and easy industrial production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
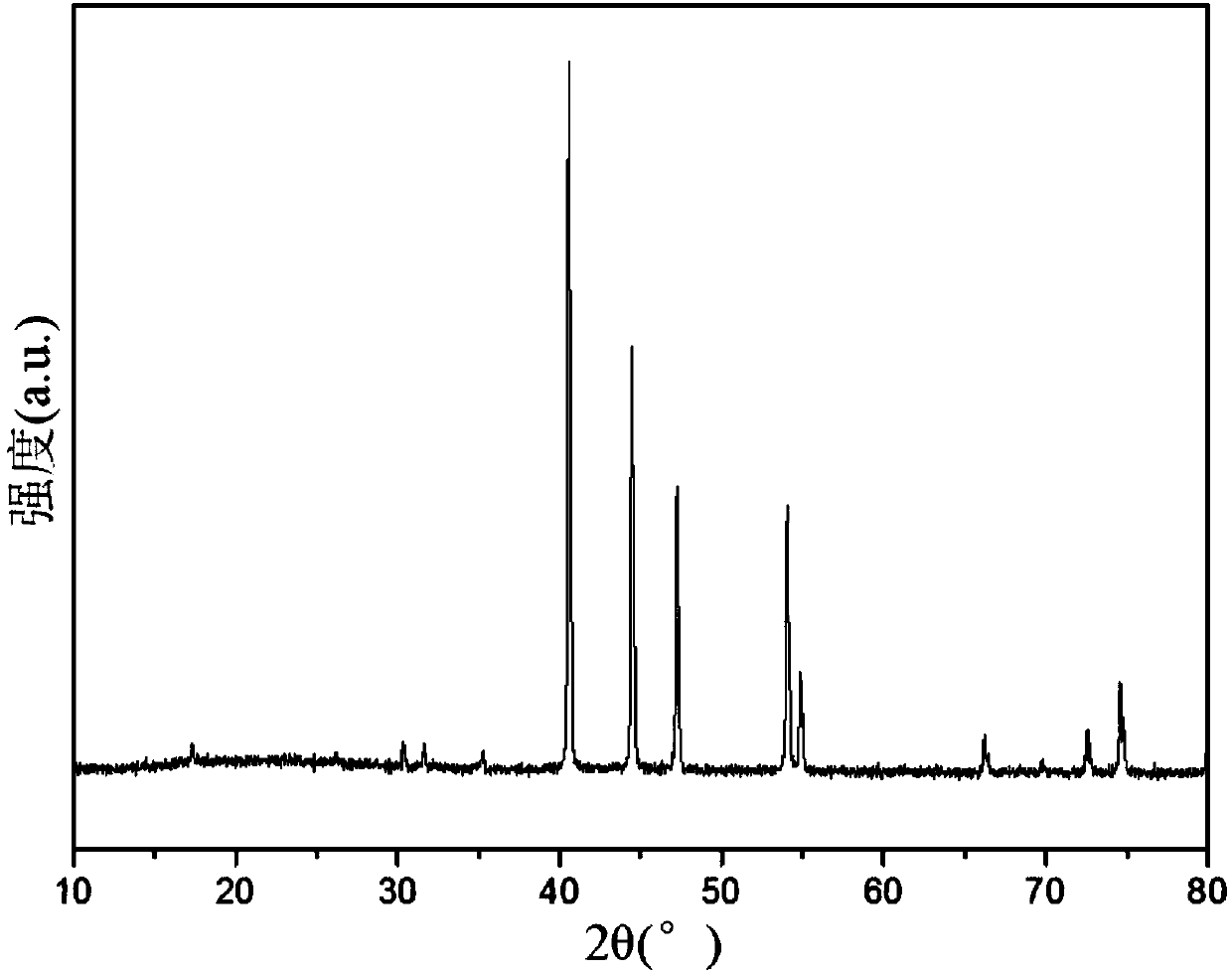
Image
Examples
specific Embodiment approach 1
[0012] Specific implementation mode one: this implementation mode is a kind of method for preparing 2-methylfuran, specifically completes according to the following steps:
[0013] A nickel phosphide catalyst is used as a catalyst to catalyze the hydrodeoxygenation of furfural to obtain 2-methylfuran.
[0014] Ni with hexagonal spatial structure 2 P has two different Ni sites and P sites, Ni(1) and Ni(2), in its bulk structural unit, where the Ni(1) site has hydrogenolysis activity, while the Ni(2) site has hydrogenation activity, make Ni 2 P catalyst surface It can be used as the hydrogenation active site of furfural hydrodeoxygenation to prepare 2-methylfuran; at the same time, Ni 2 The P-OH group on the surface of P catalyst can be used as Acid centers promote the deoxidation reaction. Therefore, in Ni 2 Metal in P center and Under the synergistic effect of the acid center P-OH, it will be able to efficiently catalyze the selective hydrodeoxygenation of furfural...
specific Embodiment approach 2
[0015] Specific embodiment two: the difference between this embodiment and specific embodiment one is: the nickel phosphide catalyst, furfural and solvent are placed in the autoclave to obtain the mixed material, and hydrogen is used to replace the air in the autoclave, and continue to pass Add hydrogen until the pressure in the autoclave reaches 0.5MPa-3.0MPa, and react with magnetic stirring at a pressure of 0.5MPa-3.0MPa, a temperature of 200-260°C and a hydrogen atmosphere for 4h-12h, and then cool to room temperature to separate phosphorus Nickel catalyst is obtained to obtain 2-methylfuran. Others are the same as the first embodiment.
[0016] This embodiment overcomes the shortcoming of Cr contamination of industrial catalysts.
[0017] The preparation process of 2-methylfuran in this embodiment is convenient;
[0018] The catalyst used in this embodiment has high activity and selectivity, the conversion rate of furfural can reach 100%, and the selectivity of 2-methyl...
specific Embodiment approach 3
[0020] Embodiment 3: The difference between this embodiment and Embodiment 2 is that the volume ratio of the furfural to the solvent is 1:(3-10). Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com