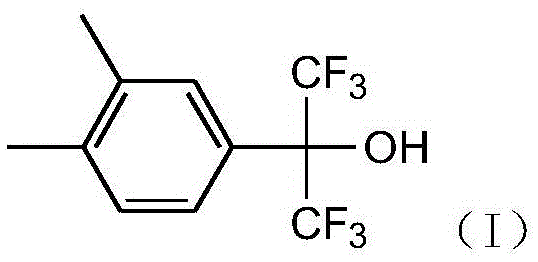
Preparation method of 2-(3,4-xylyl)-1,1,1,3,3,3-hexafluoro-2-propanol
A xylyl and o-xylene technology, which is applied in the field of preparation of 2--1,1,1,3,3,3-hexafluoro-2-propanol, can solve the problems of potential safety hazards and high reaction pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
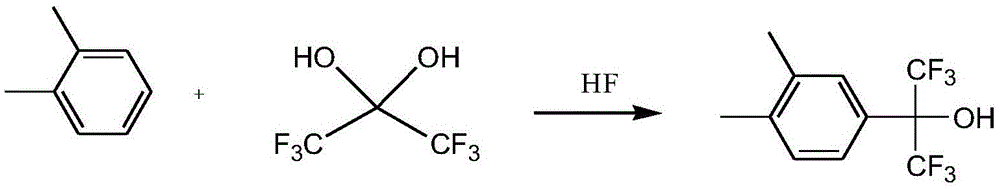
[0021] In a 500mL three-necked flask equipped with a water separator, add 110g (0.5mol) of hexafluoroacetone trihydrate and 212g (2mol) of o-xylene, separate layers, stir, and the reaction liquid is a milky white mixture, which is heated to reflux temperature, about At 94°C, water separates from the water separator (approximately 45g o-xylene is added to the water separator), and continues to heat and reflux until almost no water is released. The liquid in the reaction bottle is colorless and transparent, which is a mixture of o-xylene and hexafluoroacetone. Hydrate mixture; transfer the above-mentioned mixture of o-xylene and hexafluoroacetone monohydrate to a 1L vacuumed autoclave, add 60g of anhydrous hydrogen fluoride, raise the temperature to 85°C, and react for about 13 hours at a reaction pressure of about 4.2atm. After the reaction, lower the temperature, release the pressure, blow with nitrogen three times, wash and absorb the tail gas with water and alkali, open the k...
Embodiment 2
[0023] In a 500mL three-necked flask equipped with a water separator, add 110g (0.5mol) of hexafluoroacetone trihydrate and 106g (1mol) of o-xylene, separate layers, stir, the reaction solution is a milky white mixture, heat up to reflux temperature, and separate Water was separated from the water tank (approximately 45 g of o-xylene was added to the water separator), and the heating and reflux was continued until almost no water was released. The liquid in the reaction bottle was colorless and transparent, and was a mixture of o-xylene and hexafluoroacetone monohydrate; Transfer the above-mentioned mixture of o-xylene and hexafluoroacetone monohydrate to a 1L vacuumed autoclave, add 55g of anhydrous hydrogen fluoride, raise the temperature to 100°C, and react for about 17 hours at a reaction pressure of about 3.7 atm. After the reaction, Lower the temperature, release the pressure, purging three times with nitrogen, wash and absorb the tail gas with water and alkali, open the ...
Embodiment 3
[0025] In a 250mL three-necked flask equipped with a water separator, add 75g (0.34mol) of hexafluoroacetone trihydrate and 61g (0.58mol) of o-xylene, separate layers, stir, the reaction solution is a milky white mixture, and the temperature is raised to reflux temperature, There is water separated in the water separator (approximately 45g o-xylene is added to the water separator), continue to heat and reflux until almost no water is released, the liquid in the reaction bottle is colorless and transparent, which is a mixture of o-xylene and hexafluoroacetone monohydrate ;Transfer the mixture of o-xylene and hexafluoroacetone monohydrate above to a 1L vacuumed autoclave, add 22g of anhydrous hydrogen fluoride, heat up to 70°C, react for about 10h, and the reaction pressure is about 2.3atm. , lower the temperature, remove the pressure, purged three times with nitrogen, wash and absorb the tail gas with water and alkali, open the kettle, pour the reaction solution into ice water w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com