High-sensitivity quantitative detection kit for herpes virus 4 and herpes virus 5
A herpes virus and kit technology, applied in the field of high-sensitivity fluorescent quantitative PCR detection kits for herpes virus 4 and 5, can solve the problems of inability to report nucleic acid quantitative results, loss of nucleic acid, false negative test results, etc., and achieve optimal PCR reactions System and amplification procedures, avoid inaccurate detection results, and improve the effect of detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
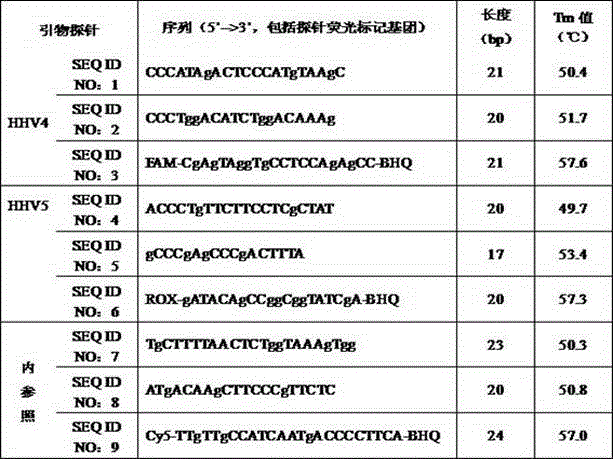
[0032] Embodiment 1: the design of primer probe of herpes virus type 4, 5 nucleic acid fluorescent PCR quantitative detection kit
[0033] According to the HHV4, HHV5, and GAPDH gene sequences queried in the NCBI GenBank database, using Vector NTI, Oligo and other primer design software, the optimally obtained primer probe sequences are shown in Table 1, and the HHV4 primers amplify the virus BamHI-W gene The above 115bp fragment, the HHV5 primer amplified is the 91bp fragment on the IE2 gene of the virus, and the internal reference primer amplifies the 144bp fragment on the human GAPDH gene.
[0034] Table 1 Design kit primer probe
[0035]
[0036] The above primers and probes were synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd.
Embodiment 2
[0037] Embodiment 2: Herpesvirus 4, 5 type nucleic acid fluorescent PCR quantitative detection kit preparation
[0038] The reaction buffer of the kit is self-prepared. According to the concentration and volume of each component in Table 2, the reaction buffer of the kit is prepared for 32 people. The prepared reaction buffer is divided into 20 μl for each reaction. After adding 10 μl of the template, the total reaction volume is 30 μl.
[0039] Table 2 The volume of each component prepared by the reaction buffer of the kit
[0040]
[0041] The negative control of the kit uses normal human negative plasma; the quantitative calibrator is the cloning plasmid containing the amplified fragments of HHV4 and HHV5, which is diluted to 4 concentrations with TE buffer as the quantitative calibrator of the kit: 5.0x10 7 copies / ml, 5.0x10 6 copies / ml, 5.0x10 5 copies / ml, 5.0x10 4 copies / ml; the positive control is Escherichia coli containing the above cloned plasmid, diluted w...
Embodiment 3
[0045] Embodiment 3: the mensuration of kit detection sensitivity
[0046] (1) Herpes virus DNA extraction
[0047] Take herpes virus type 4 and 5 positive plasma with known concentration through clinical identification, adjust the concentration of the two viruses to the same level with normal human negative plasma and mix them in equal volumes. The concentration of herpes virus type 4 and type 5 in the mixed sample is 5x10 4 copies / ml). Then use normal human negative plasma to serially dilute the mixed sample 10 times to 5x10 3 copies / ml, 5x10 2 copies / ml, 5x10 1 copies / ml, draw 400 μl each of the above-mentioned virus dilution, the negative control of the kit, and the positive control, and use the magnetic bead method virus DNA extraction reagent in Example 2 to carry out virus DNA extraction on the nucleic acid extractor, and finally each sample obtained Nucleic acid each 50 μl.
[0048] (2) Fluorescent PCR detection
[0049] Take the reaction buffer of the kit in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com