A fluorescent compound based on tetraphenylethylene and its preparation method and application
A technology of fluorescent compounds and tetraphenylethylene, which is applied in the preparation of organic compounds, chemical instruments and methods, preparation of carboxylates, etc., can solve the problems of limited application range, quenching, and weakening of fluorescence, and achieves good temperature responsiveness, Improve the effect of application scope and application environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
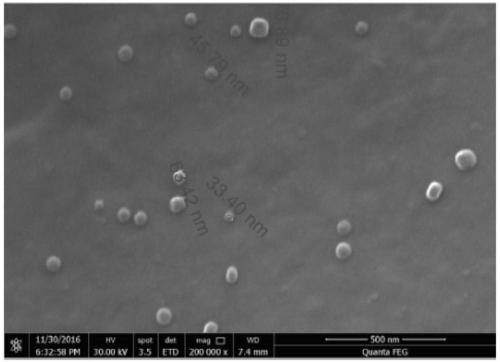
Image
Examples
Embodiment 1
[0044] Synthesis of fluorescent compounds of the present invention
[0045] The preparation route of fluorescent material of the present invention is as follows:
[0046]
[0047] Dissolve 2 mmol of compound 1 in 10 mL of dichloromethane, add thionyl chloride, the ratio of the amount of thionyl chloride to compound 1 is 5:1, add a drop of N,N-dimethylformamide, heat After reflux for 4 hours, the volatile solvent was removed by rotary evaporation under reduced pressure to obtain an acid chloride intermediate, and then anhydrous dichloromethane was added to obtain a mixed solution. The mixed solution was slowly added dropwise to 5 mL of dichloromethane solution containing 1 mmol of glyceryl monostearate and 2 mmol of triethylamine, and stirred at room temperature for 48 hours. After the reaction was completed, saturated saline was added, and then extracted with ethyl acetate. After drying with anhydrous sodium sulfate for 2 hours, the solid sodium sulfate was removed by filt...
Embodiment 2
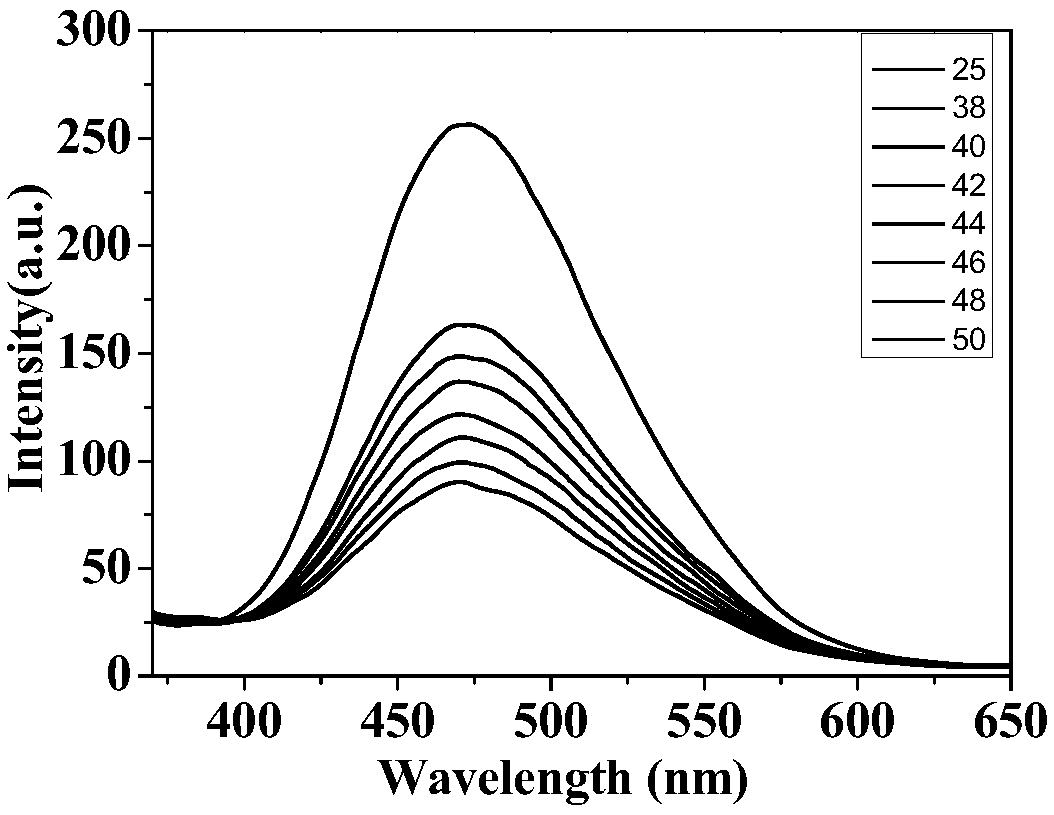
[0053] Preparation of temperature-sensitive fluorescent material with DSPC as the main raw material and the corresponding curve of fluorescence intensity versus temperature
[0054] Take 0.23mL of fluorescent compound I in chloroform, 2.0mL of DSPC in chloroform, and 0.125mL of DSPE-PEG2000 in chloroform in a 25mL round-bottomed flask, and spin evaporate for 30min under reduced pressure; then add 2.5mL of deionized water and place in a water bath Sonicate for 30 minutes and let it stand for 24 hours; take 0.2mL of nano-solution and dilute it to 2mL, measure the change of fluorescence intensity with temperature, and record the change of fluorescence intensity at 475nm with a fluorescence spectrometer. In the measurement of the change of fluorescence intensity with temperature in this embodiment, under the excitation of 325nm excitation light, the relationship diagram of the change of fluorescence intensity with temperature is as follows figure 2 shown.
Embodiment 3
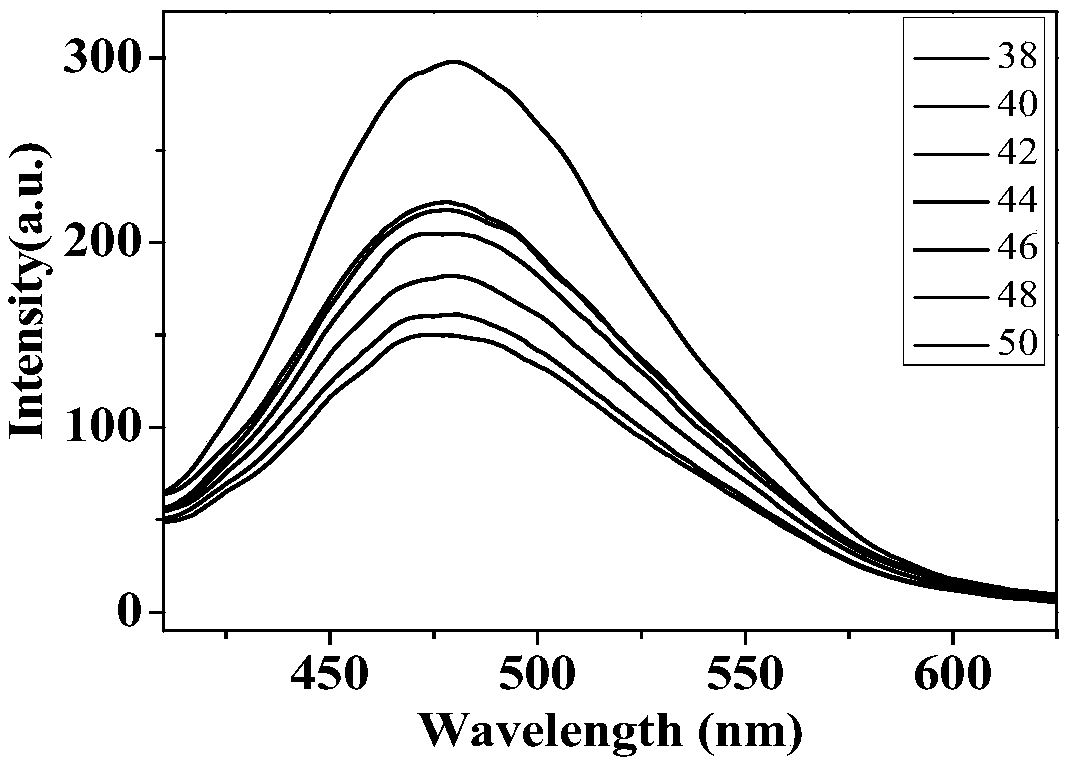
[0056] Preparation of temperature-sensitive fluorescent material with DAPC as the main raw material and the corresponding curve of fluorescence intensity versus temperature
[0057] Take 0.23mL of compound I in chloroform, 1.89mL of DAPC in chloroform, and 0.125mL of DSPE-PEG2000 in chloroform in a 25mL round-bottomed flask, and rotate under reduced pressure for 30min; then add 2.5mL of deionized water, and ultrasonically 30min, stand still for 24 hours; dilute 0.2mL of nano-solution to 2mL, measure the change of fluorescence intensity with temperature, and record the change of fluorescence intensity at 475nm with a fluorescence spectrometer. In the measurement of the change of fluorescence intensity with temperature in this embodiment, under the excitation of 325nm excitation light, the relationship diagram of the change of fluorescence intensity with temperature is as follows image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com