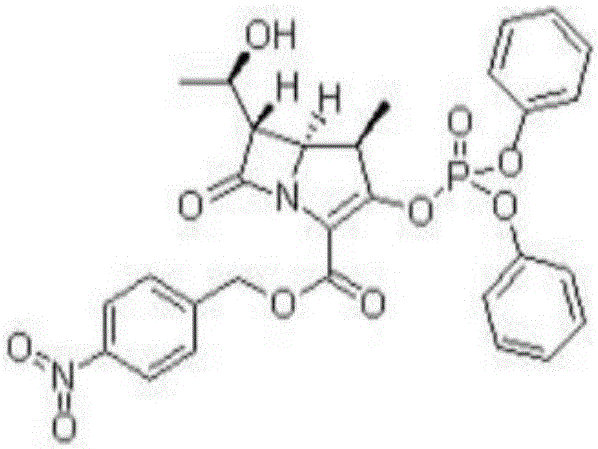
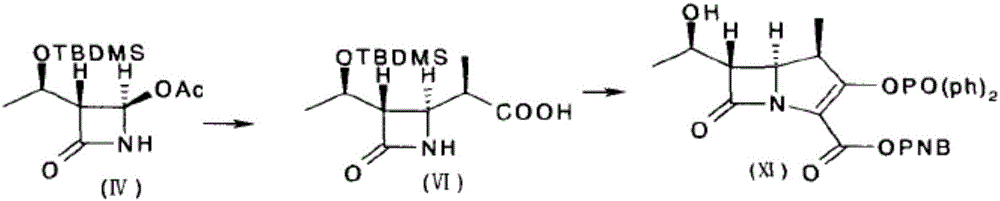
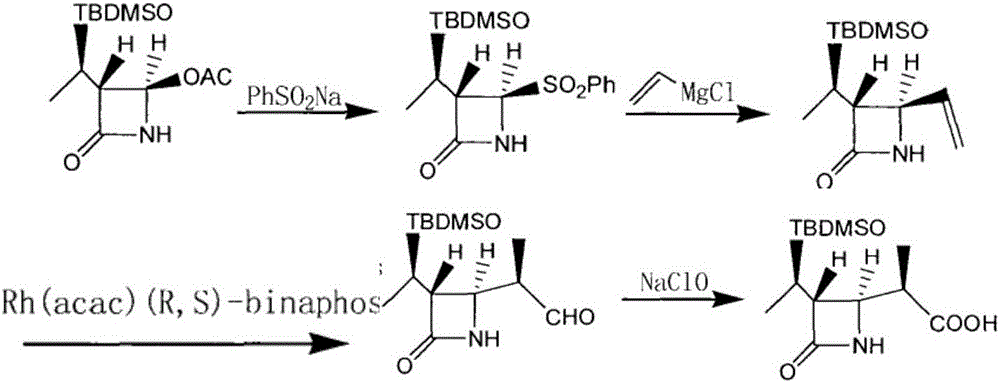
Preparation method for synthesizing key intermediate 4-BMA of 1beta-methyl carbapenem antibiotic bicyclic nucleus
A technology of methyl carbapenem and 4-BMA, applied in chemical instruments and methods, compounds of group 4/14 elements of the periodic table, organic chemistry, etc., can solve the problem of harsh conditions, many intermediate steps, and synthetic Less steps, etc., to achieve the effect of high product purity, low cost, and short reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 20g 4-AA, 40ml water, 200mlTHF and 4g of indium powder are added in the there-necked flask, and the solution of α-bromopropionamide (XV) with a large inducing group is added dropwise (α-bromopropionamide (XV) ) 32.5g, 1ml triethylamine, 50mlTHF), 40 ~ 45min dropwise, control the temperature of the reaction system 5 ~ 15 ℃, react for 30 ~ 45min, TLC detection raw material reaction is complete, filter, filter residue rinse with 20mlTHF, combine the filtrate and eluent.
[0037] Use 10% sodium bicarbonate aqueous solution to adjust the pH of the combined solution to 8, start to drop 50ml of 30% hydrogen peroxide, and complete the dropwise addition in 0.5h, keep the reaction system at 5-10°C, react for 2h, and TLC detects that the reaction of the raw materials is complete. Use 2N hydrochloric acid to adjust the pH of the reaction solution to 3, add 30ml of toluene and stir for 0.5h, and let it stand to separate into layers. The organic layer was washed twice with saturated...
Embodiment 2
[0039] 20g 4-AA, 40ml water, 120mlTHF and 2.5g of indium powder are added in the there-necked flask, and the solution of α-bromopropionamide (XV) (α-bromopropionamide (α-bromopropionamide ( XV) 32.5g, 1ml triethylamine, 50mlTHF), 40-45min dropwise, control the temperature of the reaction system at 5-15°C, react for 30-45min, TLC detects that the reaction of the raw materials is complete, filter, the filter residue is rinsed with 20mlTHF, and the filtrates are combined and rinse solution.
[0040]Use 10% sodium bicarbonate aqueous solution to adjust the pH of the combined liquid to 8, start to add 40ml of 30% hydrogen peroxide dropwise, and the dropwise addition is completed after 0.5h. Use 2N hydrochloric acid to adjust the pH of the reaction solution to 3, add 30ml of toluene and stir for 0.5h, and let it stand to separate into layers. The organic layer was washed twice with saturated brine, 60ml each time. The organic layer was cooled to 5°C, the pH of the solution was adju...
Embodiment 3
[0042] 20g 4-AA, 40ml water, 160mlTHF and 3.5g of indium powder are added in the there-necked flask, and the solution of α-bromopropionamide (XV) (α-bromopropionamide (α-bromopropionamide ( XV) 32.5g, 1ml triethylamine, 50mlTHF), 40-45min dropwise, control the temperature of the reaction system at 5-15°C, react for 30-45min, TLC detects that the reaction of the raw materials is complete, filter, the filter residue is rinsed with 20mlTHF, and the filtrates are combined and rinse solution.
[0043] Use 10% sodium bicarbonate aqueous solution to adjust the pH of the combined solution to 8, start to add 45ml of 30% hydrogen peroxide dropwise, and the dropwise addition is completed after 0.5h. Use 2N hydrochloric acid to adjust the pH of the reaction solution to 3, add 30ml of toluene and stir for 0.5h, and let it stand to separate into layers. The organic layer was washed twice with saturated brine, 60ml each time. The organic layer was cooled to 5°C, the pH of the solution was a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com