Erlotinib derivative with antitumor activity, and preparation method and application thereof
An anti-tumor activity, erlotinib technology, applied in anti-tumor drugs, organic chemistry, drug combination, etc., can solve the problems of less than 30% survival rate, high recurrence rate after treatment, and intrahepatic dissemination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] In a 500mL multi-neck flask, add 16.8g (0.1mol) of 3,4-dimethoxybenzyl alcohol, then add 150g of acetic anhydride, and slowly add 6g of nitric acid dropwise when the temperature is lowered to about 0℃. The reaction solution is slowly stirred and the addition is complete. After the temperature was raised to room temperature, after 6 hours of reaction, the acetic anhydride was evaporated under reduced pressure, 300 mL of distilled water was added, and saturated sodium carbonate solution was slowly added dropwise to adjust the pH of the reaction solution to neutral, the reaction solution was extracted three times with 200 mL of dichloromethane, and the organic Phase, the solvent was evaporated to obtain 20 g of 2-nitro-4,5-dimethoxybenzyl alcohol.
Embodiment 2
[0033]
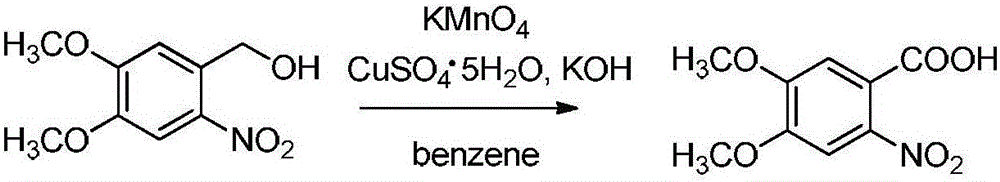
[0034] In a 500mL reaction flask with a stirrer, add 20g (0.093mol) of 2-nitro-4,5-dimethoxybenzyl alcohol to 150mL of benzene, then add 15g of potassium permanganate, and stir well. Then add 1.5 g of copper sulfate pentahydrate and 5 g of potassium hydroxide, and heat to reflux for reaction. After 10 hours of reaction, TLC monitors the completion of the raw material reaction. Pour the reaction solution into 500 mL of ice water. After stirring for 1 hour, adjust the reaction solution with glacial acetic acid. The pH was 9, the reaction solution was extracted three times with 200 mL of chloroform, the organic phases were combined, and the solvent was evaporated to obtain 13 g of 2-nitro-4,5-dimethoxybenzoic acid.
Embodiment 3
[0036]
[0037] In a 500mL reaction flask with a stirrer, add 16g of 2-nitro-4,5-dimethoxybenzoic acid to 150mL of solvent methanol, then add 105g of hydrazine hydrate and 0.4g of Raney nickel, and react under nitrogen protection. The system was heated to 50°C for reaction. After 5 hours of reaction, TLC monitored the completion of the raw material reaction. The reaction solution was filtered and the solvent methanol was evaporated to obtain 11 g of 2-amino-4,5-dimethoxybenzoic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com