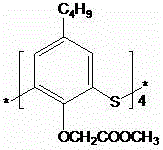
Thiacalixarene derivative with acyl hydrazone Schiff base at lower edge, and compounding method and application thereof
A technology of arene derivatives and acylhydrazone Schiff bases, which is applied in the field of thiocalix[4]arene derivatives containing acylhydrazone Schiff bases at the lower edge and their synthesis and application fields, achieving easy post-treatment, convenient operation, and high yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0018] Example: (1) Preparation of thiocalix[4]arene derivatives containing an acylhydrazone Schiff base on the lower edge
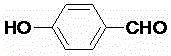
[0019] Tetraoxoacetate methyl thiocalix [4] arene derivative (1.1g, 1.0mmol) and hydrazine hydrate (2.0mL, 41.0mmol), reflux reaction in an organic solvent, stop after 48 hours, after the end of the reaction, cool When it is room temperature, the solvent is distilled and spin-dried under reduced pressure, and the product is dissolved in dichloromethane, washed with water, and the organic layer is spin-dried to obtain a thick product. After recrystallization, a white solid is obtained, and the yield is 81%; the white solid (0.4 g, 2.0mmol) was heated and dissolved in an organic solvent, p-hydroxybenzaldehyde (0.268g, 2.2mmol) was slowly added therein, heated to reflux, solids were generated in about ten minutes, continued to stir for 12 hours, suction filtered, absolute ethanol Wash until the filtrate is clarified to obtain a pure solid product, which is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com