Xanthone sulfonamide derivative as well as preparation method and application thereof
A technology of xanthone sulfonamide and derivatives, applied in the field of compounds, can solve the problems of difficult synthesis, high cost, low yield, etc., and achieve the effects of strong α-glucosidase inhibitory activity and good drug selection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
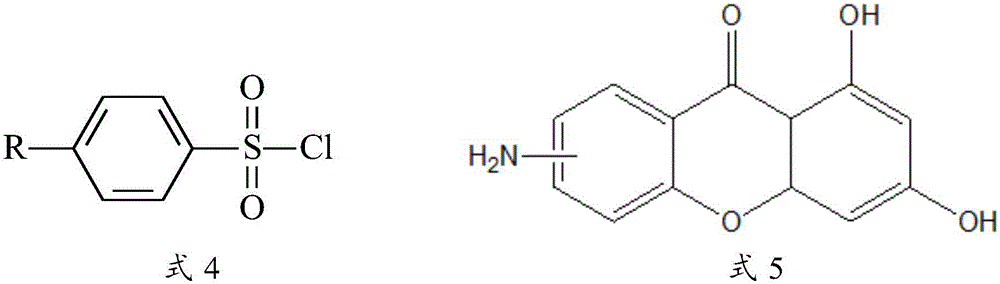
[0085] Embodiment 1: Compound 6-Me and its synthesis
[0086] Mix excess p-toluenesulfonyl chloride (1.25g, 6.58mmol, 16 equivalents) with 0.100g (0.41mmol) of 6-amino-1,3-dihydroxy-9H-xanthene-9-one in anhydrous Add 10 mL of dry redistilled pyridine under anaerobic conditions, and react at room temperature for 12 hours. Pour the reaction mixture into hydrochloric acid, dissolve the solid in 20 mL of methanol after suction filtration, add 3 mL of 1M sodium hydroxide solution to reflux for 2 hours, remove most of the solvent, pour the system into saturated sodium chloride solution, suction filter, dry, column chromatography. 94 mg of the product was obtained with a yield of 58%.
[0087] 1 H NMR spectrum (solvent DMSO-d 6 , TMS internal standard, ppm) δ12.83(s, 1H), 11.22(s, 1H), 11.09(s, 1H), 7.98(d, J=8.5Hz, 1H), 7.79(d, J=7.8Hz , 2H), 7.40 (d, J = 7.9Hz, 2H), 7.18 (d, J = 8.7Hz, 2H), 6.38 (s, 1H), 6.19 (s, 1H), 2.34 (s, 3H).
Embodiment 2
[0088] Embodiment 2: Compound 6-H and its synthesis
[0089] Mix excess benzenesulfonyl chloride (1.26mL, 10.0mmol, 24.4eq) with 0.100g (0.41mmol) of 6-amino-1,3-dihydroxy-9H-xanthene-9-one, the reaction conditions and synthesis method are the same as Embodiment 1, obtain product 135mg, productive rate 86.5%.
[0090] 1 H NMR spectrum (solvent DMSO-d 6 , TMS internal standard, ppm) δ12.81(s, 1H), 11.31(s, 1H), 11.05(s, 1H), 7.99(d, J=8.5Hz, 1H), 7.91(d, J=7.6Hz , 2H), 7.63 (m, 3H), 7.20 (s, 1H), 6.18 (d, J=2.2Hz, 1H).
Embodiment 3
[0091] Embodiment 3: Compound 6-OMe and its synthesis
[0092] Mix excess p-methoxybenzenesulfonyl chloride (1.1g, 5.36mmol, 13 equivalents) with 0.100g (0.41mmol) of 6-amino-1,3-dihydroxy-9H-xanthene-9-one, the reaction conditions 1. The synthetic method is the same as in Example 1, and 84 mg of the product is obtained, with a yield of 49.6%.
[0093] 1 H NMR spectrum (solvent DMSO-d 6 , TMS internal standard, ppm) 1 H NMR (400MHz, DMSO-d 6 )δ12.83(s,1H),11.18(s,1H),11.05(s,1H),7.98(d,J=8.5Hz,1H),7.84(d,J=9.0Hz,2H),7.17( s, 2H), 7.11 (d, J=9.0Hz, 2H), 6.37 (d, J=2.1Hz, 1H), 6.18 (d, J=2.1Hz, 1H), 3.80 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com