A kind of preparation method of S-1 composition
A technology for a composition and a solid composition, which is applied in the directions of pharmaceutical combinations, active ingredients of heterocyclic compounds, and medical preparations of non-active ingredients, etc. problems, to achieve the effects of rapid and complete drug dissolution, simple preparation process and reduced contact degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 5
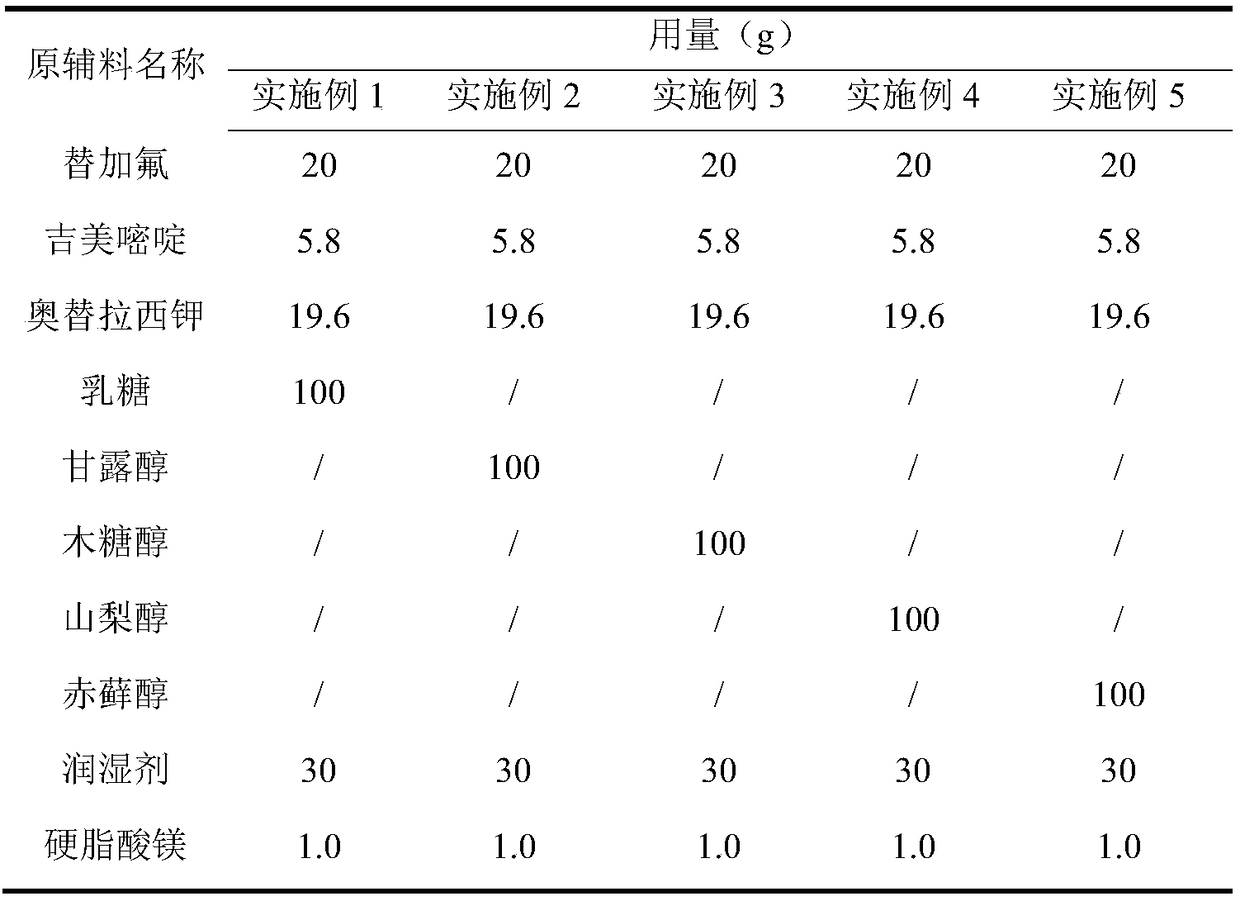
[0036] Preparation process: According to the proportions in Table 1, pass the raw and auxiliary materials through an 80-mesh sieve, mix gimeracil, oteracil potassium and fillers evenly, add purified water, use a Glatt wet granulator to granulate, dry at 60°C, and dry at 30°C Mesh sieve and dry the whole grain, add tegafur and magnesium stearate, mix evenly, fill capsules, and prepare S-1 capsules.
[0037] Table 1
[0038]
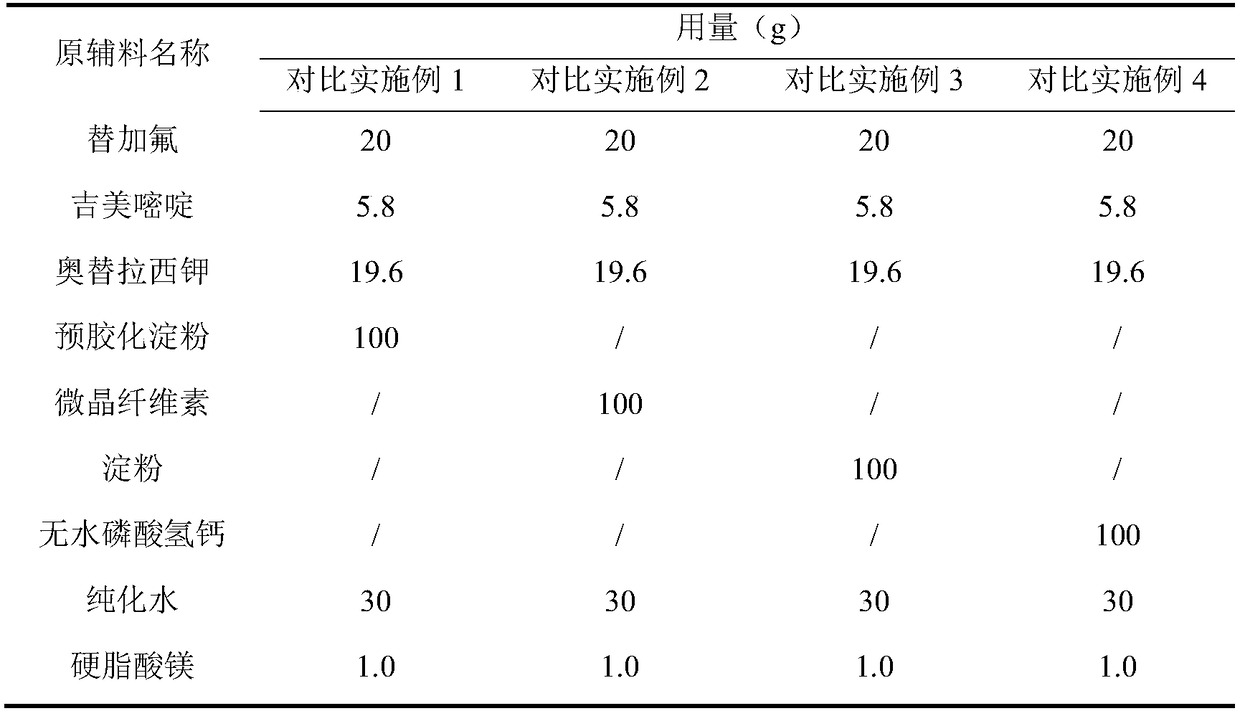
Embodiment 1~5 and comparative Embodiment 1~4
[0047] Embodiments 1-5 and comparative examples 1-4: dissolution rate determination
[0048]Adopt dissolution measurement method (Chinese Pharmacopoeia 2015 edition general rule 0931 second method), get the capsule in embodiment 1~5 and comparative example 1~4, take purified aqueous solution as dissolution medium, rotating speed is 50 rpm, temperature is 37±0.5°C, operate according to the law, at 15 minutes, take 10ml of the eluate, filter the eluate with a 0.45μm filter membrane, and use high performance liquid chromatography to determine tegafur, gimeracil and oteracil in S-1 capsules The dissolution rate of potassium is limited to 85% of the labeled amount, and the dissolution results are shown in Table 4.
[0049] Table 4
[0050]
[0051]
[0052] The results of the dissolution test show that the dissolution rate of the active ingredients in Examples 1 to 5 (the filler is lactose or sugar alcohols) is greater than 85% in 15 minutes, and the dissolution rate is rapi...
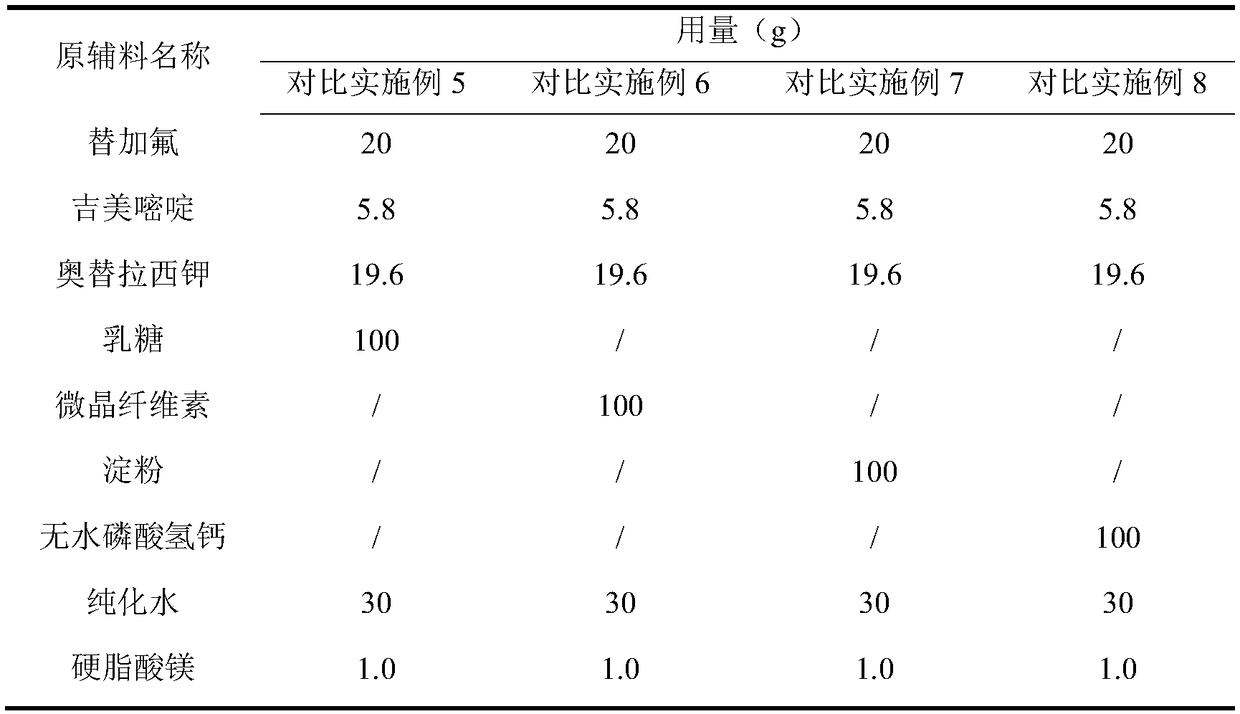
Embodiment 1~5 and comparative Embodiment 5~8
[0053] Embodiment 1~5 and comparative example 5~8: preparation stability investigation
[0054] (1) Influencing factor test: get an appropriate amount of Sigion Capsules in Examples 1 to 5 and Comparative Examples 5 to 8, place them under strong light irradiation (4500lx ± 500lx), high temperature (60°C, high humidity 90%) Placed under the same conditions, samples were taken to check the relevant substances on the 5th and 10th days respectively, and the results are shown in Table 5:
[0055] table 5
[0056]
[0057] (2) Accelerated test: get the Tigio capsules in Examples 1 to 5 and Comparative Examples 5 to 8, place 40°C of temperature under commercially available packaging, and place 6 capsules in a constant temperature and humidity box with a relative humidity of 75%. month, and at the end of the 1st, 2nd, 3rd, and 6th months, the relevant substances were sampled and inspected. The results are shown in Table 6:
[0058] Table 6
[0059]
[0060] Influencing factor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com