Complex calcium-based lubricating grease and preparing method thereof
A complex calcium-based, grease technology, applied in the field of grease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
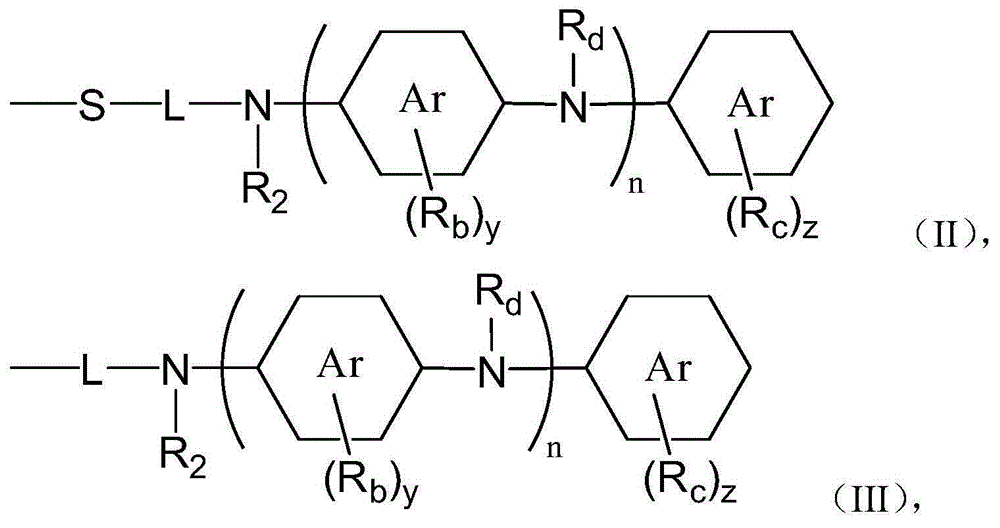
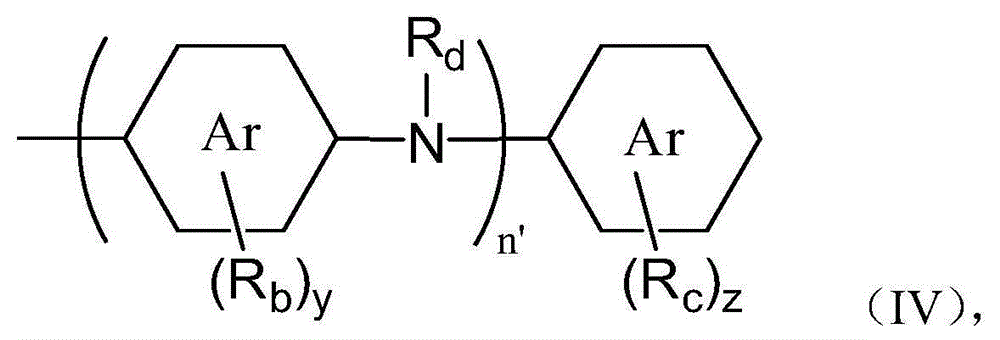
[0134] The preparation method according to the present invention comprises the first step of reacting the phenol compound represented by the general formula (X) and the amine compound represented by the general formula (Y) in the presence of the aldehyde compound represented by the general formula (Z). step.
[0135]
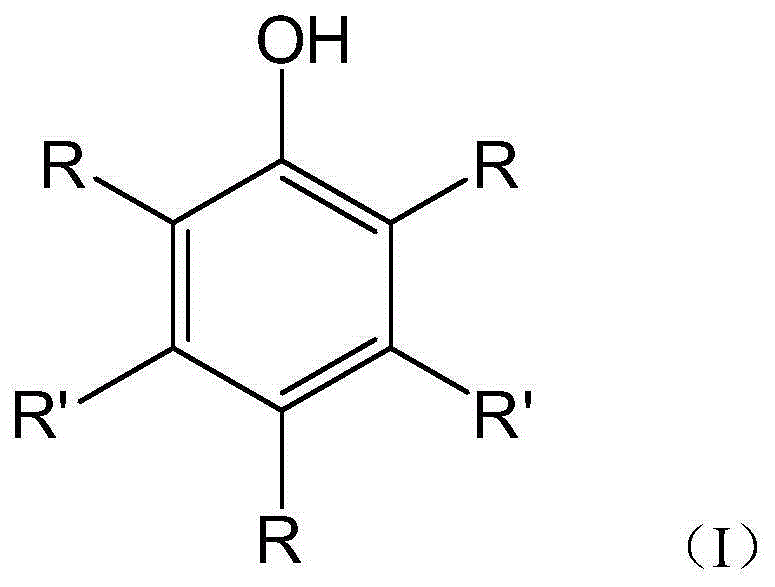
[0136] According to the present invention, in the general formula (X), when there are multiple groups, each group R 0 The same or different from each other, each independently selected from hydrogen, -SH and C 1-300 Straight or branched chain alkyl, provided that at least one group R 0 Yes-SH.
[0137] According to the present invention, in the general formula (X), as the C 1-300Straight chain or branched chain alkyl, such as C 1-20 Straight chain or branched chain alkyl (preferably C 1-10 Straight chain or branched chain alkyl, more preferably C 1-4 straight-chain or branched-chain alkyl) or polyolefin-based. Specific examples of the polyolefin group ...
Embodiment 1
[0208] The preparation of shielding phenol compound (structure sees the following formula):
[0209]
[0210] Under nitrogen protection atmosphere, in the 250ml four-neck flask that stirrer, thermometer, condenser tube and dropping funnel are equipped with, add 31.65 grams (133mmol) 2,6-di-tert-butyl-4-mercaptophenol, 1.86 grams (62mmol ) formaldehyde, 28.15 grams (153 mmol) of N-phenyl-p-phenylenediamine, 0.75 grams (7.5 mmol) of hydrochloric acid and 150 mL of isopropanol, stirred rapidly, and reacted at 25 ° C for 24 h. After the reaction was finished, the solvent and the generated small amount of water were distilled off under reduced pressure, and separated by column chromatography to obtain the masked phenol product with the above structure.
[0211] The characterization data of this product are as follows:
[0212] 1 H NMR (300MHz, CDCl 3 ): δ1.36-1.54(18H), 3.75(1H), 4.80(2H), 5.32(1H), 6.80(2H), 6.97(5H), 7.17(2H), 7.26(2H), 7.55(1H) ;
[0213] 13 C NMR (75MH...
Embodiment 2
[0220] The preparation of shielding phenol compound (structure sees the following formula):
[0221]
[0222] Under a nitrogen protection atmosphere, in a 250ml four-necked flask equipped with a stirrer, a thermometer, a condenser and a dropping funnel, add 8.57 grams (36mmol) of 2,6-di-tert-butyl-4-mercaptophenol, 0.45 grams (15mmol ) formaldehyde, 10.14 g (39 mmol) of N,N'-diphenyl-1,4-phenylenediamine and 150 mL of toluene, stirred rapidly, and reacted at 90° C. for 4 h. After the reaction was finished, the solvent and the generated small amount of water were distilled off under reduced pressure, and separated by column chromatography to obtain the masked phenol product with the above structure.
[0223] The characterization data of this product are as follows:
[0224] 1 H NMR (300MHz, CDCl 3 ): δ1.36(18H), 5.23(2H), 6.80-7.02(10H), 7.17(2H), 7.26(4H), 7.55(1H);
[0225] 13 C NMR (75MHz, CDCl 3 ): δ29.6, 34.6, 55.2, 117.1, 121.8, 126.2, 129.5, 136.6, 143.6, 153.4;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com