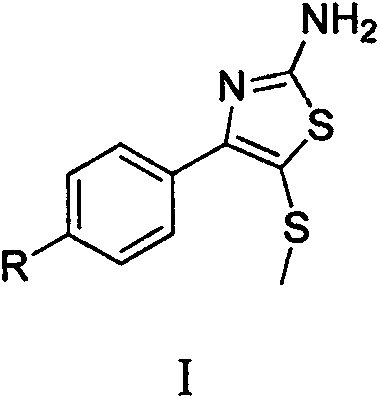
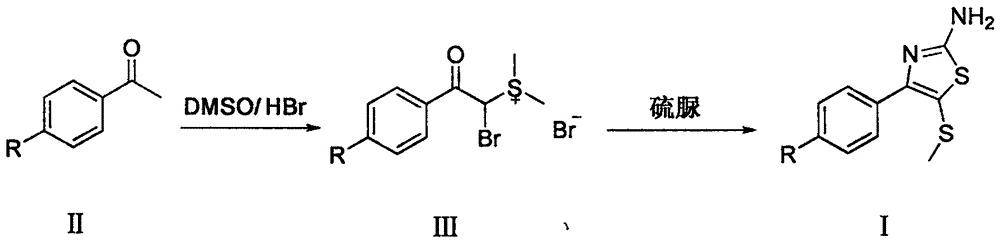
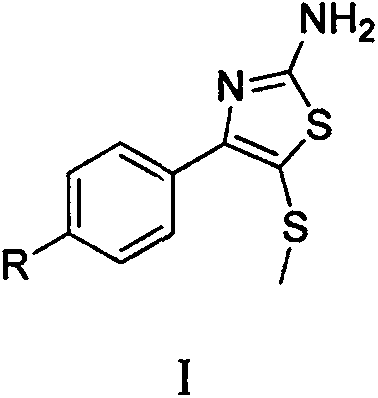
Synthetic method of 2-amino-4-aryl-5-methylthiothiazole compound
A technology of methylthiothiazole and compounds, which is applied in the field of organic chemical synthesis, can solve the problems of poisonous and harmful use, low yield, high cost, etc., and achieve the effects of mild reaction conditions, short process lines, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: Synthesis of 2-amino-4-(p-nitrophenyl)-5-methylthiothiazole
[0018] (a) Weigh p-nitroacetophenone (6.52g, 39.5mmol), dissolve it in a round bottom flask adding 40% hydrobromic acid (20ml) and dimethyl sulfoxide (20ml) mixture, seal it, avoid Light, the mixture was heated to 40°C, reacted for 10 hours, and cooled. Add ether (20ml) and isopropanol (20ml) to a round bottom flask, refrigerate and stand overnight, a large amount of precipitate precipitates, filter, wash with dichloromethane, and dry to obtain α-bromo-p-nitroacetophenone dimethyl Sulfonium salt, white crystal, weighing 12.16g, yield 80%, melting point 119-123°C.
[0019] (b) Take the sulfonium salt (0.5g, 1.3mmol) prepared by the above steps, add water (5ml) to dissolve, add thiourea (0.1g, 1.3mmol), stir at room temperature for 3 hours, then add ethanol (5ml), and heat up to React at 90°C for 12 hours. Cool in an ice-water bath, store in refrigerator overnight, precipitate out, filter with ...
Embodiment 2
[0020] Embodiment 2: Synthesis of 2-amino-4-(p-chlorophenyl)-5-methylthiothiazole
[0021] (a) Weigh p-chloroacetophenone (6.11g, 39.5mmol) in a round bottom flask, add dimethyl sulfoxide (10ml) and hydrobromic acid (20ml), seal it, avoid light, and heat the mixture to 60°C, react for 8 hours, and cool. Add ether (20ml) and isopropanol (20ml) to a round bottom flask, refrigerate and stand overnight, a large amount of precipitate precipitates, filter, wash with dichloromethane, and dry naturally to obtain α-bromo-p-chloroacetophenone dimethyl Sulfonium salt, white crystal, weighing 10.35g, yield 70%, melting point 124-128°C.
[0022] (b) Take the sulfonium salt (0.50g, 1.3mmol) obtained in step (a), add water (10ml) to dissolve, add thiourea (0.2g, 2.6mmol), stir at room temperature for 3 hours, add ethanol (5ml), The temperature was raised to 70° C. for 10 hours. After cooling, it was left to stand in the refrigerator overnight, and no precipitate was precipitated. It was e...
Embodiment 3
[0023] Example 3: Synthesis of 2-amino-4-(p-fluorophenyl)-5-methylthiothiazole
[0024] (a) Weigh p-fluoroacetophenone (5.45g, 39.5mmol), dissolve it in a round-bottom flask adding 40% hydrobromic acid (20ml) and dimethyl sulfoxide (20ml) mixture, seal it, and avoid light , The mixture was heated to 30°C, reacted for 12 hours, and cooled. Add ether (20ml) and isopropanol (20ml) to a round bottom flask, refrigerate and stand overnight, precipitate precipitate, filter, wash with dichloromethane, dry to obtain α-bromo-p-fluoroacetophenonyl dimethyl Sulfonium salt, white crystal, weighing 8.49g, yield 60%, melting point 120-124°C.
[0025] (b) Weigh the α-bromo-p-fluoroacetophenonyl dimethylsulfonium salt (0.50g, 1.4mmol) obtained in step (a), add water (10ml) to dissolve, add thiourea (0.55g, 7.2 mmol), stirred at room temperature for 3 hours, then added ethanol (5 ml), and heated to 60°C for 14 hours. Cool and store in refrigerator overnight, no precipitate was precipitated, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com