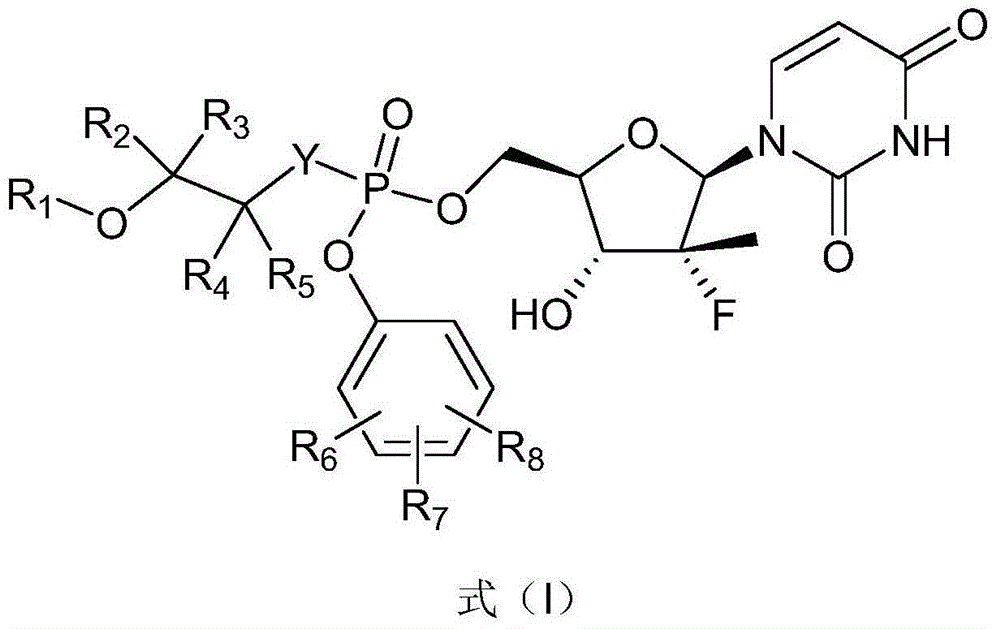
Nucleoside phosphamide compound and preparation method and application of nucleoside phosphamide in medicine
A compound and hydrate technology, applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of poor drug efficacy and aggravate the burden on the kidneys, and achieve the effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0297] (S)-Isopropyl(((((2R,3R,4R,5R)-5-(2,4-dicarbonyl-3,4-dihydropyrimidin-1(2H)-yl)-4-fluoro -3-Hydroxy-4-methyltetrahydrofuran-2-yl)methoxy)(2,3-dihydrobenzofuran-6-oxyl)phosphoryl)amino)propionate (K1) and its isomers body preparation
[0298]
[0299] Preparation of intermediate compound (S)-isopropyl-2-(((pentafluorophenoxy)(2,3-dihydrobenzofuran-6-oxyl)phosphoryl)amino)propionate
[0300] Dissolve phosphorus oxychloride (1.53g, 10mmol) in dichloromethane (10mL), cool to -60°C, slowly add 6-hydroxy-2,3-dihydrobenzofuran (1.36g, 10mmol) and tris Ethylamine (1.01 g, 10 mmol) in dichloromethane (10 mL). After the dropwise addition was completed, the cooling bath was removed, and the mixture was raised to 25°C and stirred for 2 hours. The reaction system was further cooled to -60°C, L-alanine isopropyl ester hydrochloride (1.51 g, 9 mmol) was added, and then a dichloromethane solution (5 mL) of triethylamine (2.53 g, 25 mmol) was added dropwise. After the dropwise ad...
Embodiment 2
[0321] (S)-Isopropyl-2-((S)-((((2R,3R,4R,5R)-5-(2,4-dicarbonyl-3,4-dihydropyrimidine-1(2H) -yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-yl)methoxy)(benzofuran-6-oxyl)phosphoryl)amino)propionate (K2-b) and (S)-Isopropyl-2-((R)-((((2R,3R,4R,5R)-5-(2,4-dicarbonyl-3,4-dihydropyrimidine-1(2H) -yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-yl)methoxy)(benzofuran-6-oxyl)phosphoryl)amino)propionate (K2-a) preparation
[0322] Except that 6-hydroxybenzofuran was used instead of 6-hydroxy-2,3-dihydrobenzofuran, and the dosage of 6-hydroxybenzofuran was 0.134g, other operations were performed in the same manner as in Example 1 to obtain the title compound . Among them, K2-b is 25 mg, and K2-a is 8 mg.
[0323]
[0324] Its structure is characterized as follows:
[0325] ESI-MS: m / z 570.2 (M + +H)
[0326] 1 H NMR (DMSO-d 6 ,400MHz)δ11.54(s,1H),7.99(s,1H),7.64-7.51(m,3H),7.15(d,J=8.0Hz,1H),6.96(s,1H),6.14-5.90 (m,3H),5.54(d,J=8.0Hz,1H),4.83(m,1H),4.39-3.83(m,5H),1.28-...
Embodiment 3
[0334] (S)-Isopropyl-2-((S)-((((2R,3R,4R,5R)-5-(2,4-dicarbonyl-3,4-dihydropyrimidine-1(2H) -yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-yl)methoxy)(benzo[b][1,4]dioxane-5-oxyl)phosphoryl) Amino)propionate (K3-b) and (S)-isopropyl-2-((R)-((((2R,3R,4R,5R)-5-(2,4-dicarbonyl-3 ,4-dihydropyrimidin-1(2H)-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-yl)methoxy)(benzo[b][1,4]diox Preparation of hexylcyclo-5-oxy)phosphoryl)amino)propionate (K3-a).
[0335] Except using 2,3-dihydro-5-hydroxy-benzo[b][1,4]dioxane instead of 6-hydroxy-2,3-dihydrobenzofuran, making 2,3-dihydro- Except that the amount of 5-hydroxy-benzo[b][1,4]dioxane was 0.137 g, the same operations as in Example 1 were performed to obtain the title compound. Among them, K3-b is 15 mg, and K3-a is 8 mg.
[0336]
[0337] Its structure is characterized as follows:
[0338] ESI-MS: m / z 588.3 (M + +H)
[0339] 1 H NMR (DMSO-d 6 ,400MHz)δ11.52(s,1H),7.58(s,1H),6.86(m,2H),6.74-6.70(m,1H),6.08-6.05(m,1H),5.9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ec50 | aaaaa | aaaaa |
| Ic50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com