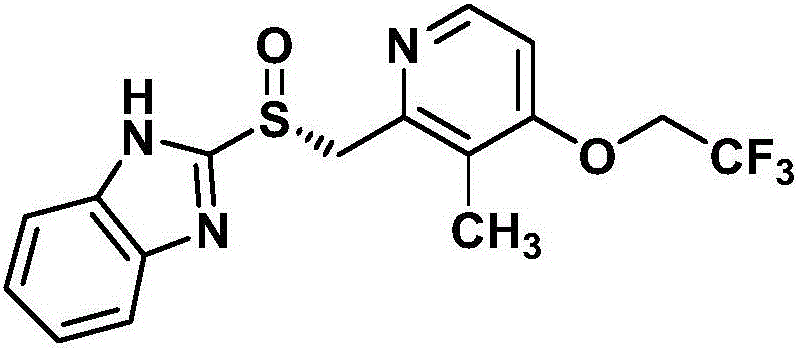
Preparation method for dexlansoprazole
A technology of dexlansoprazole and benzimidazole, which is applied in the field of preparation of dexlansoprazole, can solve problems such as increased by-products, slow reaction speed, impact on final product yield and purity, and achieves guaranteed product yield , improve product quality, shorten the effect of response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A preparation method of dexlansoprazole, comprising the following steps:
[0029] (1) Preparation of 2-chloromethyl-3-methyl-4-(2,2,2-trifluoroethoxy)pyridine
[0030] At room temperature, 2-hydroxymethyl-3-methyl-4-(2,2,2-trifluoroethoxy)pyridine (250g, 1.13mol) in dioxane (2.5L) was added Sulfoxide (0.11 L, 1.47 mol). The mixture was stirred and reacted at 50 °C for 3 h, then cooled to room temperature. Dilute with dichloromethane and wash successively with saturated sodium bicarbonate solution and brine solution. Dry over anhydrous magnesium sulfate, filter, and evaporate the solvent under reduced pressure to obtain 245 g of 2-chloromethyl-3-methyl-4-(2,2,2-trifluoroethoxy)pyridine with a yield of 90.5%. 1 H-NMR (CDCl 3 , 300MHz) δ8.34(d, J=5.6Hz, 1H), 6.67(d, J=5.6Hz, 1H), 4.68(s, 2H), 4.38(q, J=7.8Hz, 2H), 2.31( s, 3H).
[0031] (2) Preparation of 2-[[2-(3-methyl-4-(2,2,2-trifluoroethoxy)pyridyl)methyl]-sulfanyl]-1H-benzimidazole
[0032] 2-Chloromethyl-3-me...
example 1
[0034] Example 1. Under nitrogen protection, 2-[[2-(3-methyl-4-(2,2,2-trifluoroethoxy)pyridyl)methyl]-sulfanyl]-1H-benzimidazole (50.0g, 0.142mol), toluene (250mL) and L-(+)-diethyl tartrate (5.5mL, 0.038mol) were mixed and stirred at 50°C-55°C for 30 minutes. Under nitrogen protection, titanium tetraisopropoxide (4.15 mL, 0.0143 mol) was added, and the mixture was stirred at 50°C-55°C for 1 hour. Diisopropylethylamine (8.13 mL, 0.048 mol) was added under cooling, 82% cumene peroxide (76.5 mL, 0.425 mol) was added dropwise at 0° C. to 10° C., and the mixture was incubated and stirred for 3.5 hours.
[0035] The reaction solution was detected by HPLC (CHIRALCEL OD, mobile phase: n-hexane / ethanol=90 / 10, flow rate: 1.0 mL / min, detection wavelength: 285 nm): sulfide 1.26%, sulfone 1.01%. No other related substances were detected. Enantiomer detection: 98.0% ee.
example 2
[0036] Example 2. Under nitrogen protection, 2-[[2-(3-methyl-4-(2,2,2-trifluoroethoxy)pyridyl)methyl]-sulfanyl]-1H-benzimidazole (4.5kg, 12.7mol), toluene (22L) and L-(+)-diethyl tartrate (958mL, 5.6mol) were mixed. Under the protection of nitrogen, titanium tetraisopropoxide (7.47 L, 2.53 mol) was added at 50° C. to 60° C., and the mixture was kept stirring for 30 minutes. Diisopropylethylamine (0.733 L, 4.44 mol) was added at room temperature, and 82% cumene peroxide (6.88 L, 37.5 mol) was added dropwise at -5°C to 5°C, and kept stirring for 1.5 hours. In the above reaction solution, add 30% sodium thiosulfate aqueous solution to decompose excess cumene peroxide. Separate the organic phase, add water (4.5 L), petroleum ether (60-90° C.) (13.5 L), tert-butyl methyl ether (18 L) and petroleum ether (27 L) in sequence, and stir to crystallize. Filter and wash with tert-butyl methyl ether-toluene (4:1) (4L) to obtain dexlansoprazole.
[0037] Use HPLC to detect crystallizatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com