Allyl acetate hydroformylation method
A technology of propylene acetate hydroformyl and hydroformyl, applied in the field of formylation reaction, can solve problems such as reduction, and achieve the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
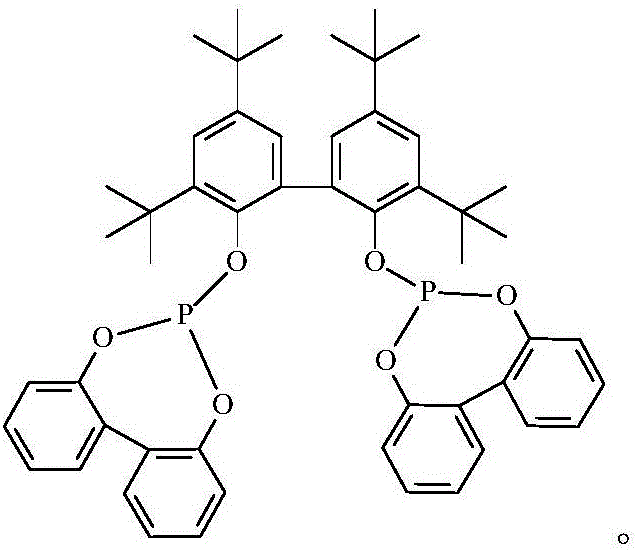
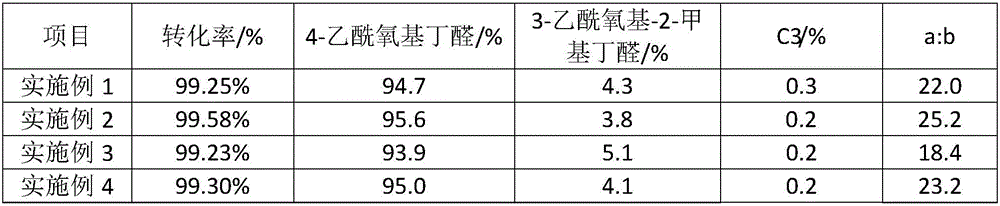
[0023] Add rhodium acetylacetonate dicarbonyl (0.08mmol) and bisphosphine-I ligand (0.48mmol) into a 300mL autoclave, replace the atmosphere in the autoclave with nitrogen three times, and then add 100g of toluene. Syngas (CO / H 2 The volume ratio is 1:1) to replace the atmosphere in the kettle three times, then raise the temperature to 70°C, add 17.6g of propylene acetate, and pressurize with synthesis gas to a pressure (gauge pressure) of 1.2MPa. Keep the pressure of the reactor at 1.2MPa, monitor the reaction of the gas, and when the gas does not react further, cool the reactor and release the gas. The reaction solution was analyzed by gas chromatography, and the results are shown in Table 1.
Embodiment 2
[0025] Add rhodium acetylacetonate dicarbonyl (0.06mmol) and bisphosphine-I ligand (0.60mmol) into a 300mL autoclave, replace the atmosphere in the autoclave with nitrogen three times, and then add 100g of n-heptane. Syngas (CO / H 2 The volume ratio is 1:1) to replace the atmosphere in the kettle three times, then raise the temperature to 75°C, add 13.6g of propylene acetate, and pressurize with synthesis gas to a pressure (gauge pressure) of 1.0MPa. Keep the pressure of the reactor at 1.0 MPa, monitor the reaction of the gas, and when the gas does not react further, cool the reactor and release the gas. The reaction solution was analyzed by gas chromatography, and the results are shown in Table 1.
Embodiment 3
[0027] Add rhodium acetylacetonate dicarbonyl (0.08mmol) and bisphosphine-I ligand (0.40mmol) into a 300mL autoclave, replace the atmosphere in the autoclave with nitrogen three times, and then add 100g of toluene. Syngas (CO / H 2 The volume ratio is 1:1) to replace the atmosphere in the kettle three times, then raise the temperature to 65°C, add 6.8g of propylene acetate, and pressurize with synthesis gas to a pressure (gauge pressure) of 1.5MPa. Keep the pressure of the reactor at 1.5MPa, monitor the reaction of the gas, and when the gas does not react further, cool the reactor and release the gas. The reaction solution was analyzed by gas chromatography, and the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com