Compound sildennafil dapoxetine slow-release capsule and preparation method thereof
A technology of sildenafil and sustained-release capsules, applied in the directions of pharmaceutical formulations, bulk delivery, drug delivery, etc., can solve the problems of different half-lives and the inability to exert drug effects at the same time, and achieve the effect of treating impotence and premature ejaculation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The present invention specifically describes the materials and test methods used in the test. While many of the materials and procedures used in the experiments are well known in the art, the present invention is described here in as much detail as possible. Hereinafter, materials and operating methods used in the present invention are well known in the art unless otherwise specified. Example 1: Preparation of Compound Sildenafil Dapoxetine Sustained-release Capsules
[0026] Prescription of Dapoxetine Granules:
[0027] Dapoxetine Hydrochloride 67.16g Hydroxypropylmethylcellulose 100g microcrystalline cellulose 30g lactose 37.84g povidone (10% solution) 5g
[0028] Prescription for Sildenafil Granules:
[0029] Sildenafil Citrate 56.2g dry starch 15g microcrystalline cellulose 83.8g povidone (10% solution) 5g
[0030] a. Preparation of dapoxetine sustained-release granules:
[0031] Weigh the ...
Embodiment 2
[0036] Example 2: Preparation of Compound Sildenafil Dapoxetine Sustained-release Capsules
[0037] Prescription of Dapoxetine Granules:
[0038] Dapoxetine Hydrochloride 67.16g Hydroxypropylmethylcellulose 80g Ethyl cellulose 20g microcrystalline cellulose 30g lactose 37.84g povidone (10% solution) 5g
[0039] Prescription for Sildenafil Granules:
[0040] Sildenafil Citrate 56.2g Croscarmellose Sodium 15g microcrystalline cellulose 83.8g povidone (10% solution) 5g
[0041] With reference to the formula and method of Example 1 of the present invention, the only difference is that the type and content of auxiliary materials have been changed. In the obtained compound sildenafil dapoxetine sustained-release capsules, each capsule contains 50 mg of sildenafil and 60 mg of dapoxetine.
Embodiment 3
[0042] Example 3: Preparation of Compound Sildenafil Dapoxetine Sustained-release Capsules
[0043] Prescription of Dapoxetine Granules:
[0044] Dapoxetine Hydrochloride 67.16g Hydroxypropylmethylcellulose 100g Ethyl cellulose 20g microcrystalline cellulose 10g lactose 37.84g povidone (10% solution) 5g
[0045] Prescription for Sildenafil Granules:
[0046] Sildenafil Citrate 56.2g Croscarmellose Sodium 10g Sodium carboxymethyl starch 5g microcrystalline cellulose 83.8g povidone (10% solution) 5g
[0047] With reference to the formula and method of Example 1 of the present invention, the only difference is that the type and content of auxiliary materials have been changed. In the obtained compound sildenafil dapoxetine sustained-release capsules, each capsule contains 50 mg of sildenafil and 60 mg of dapoxetine.
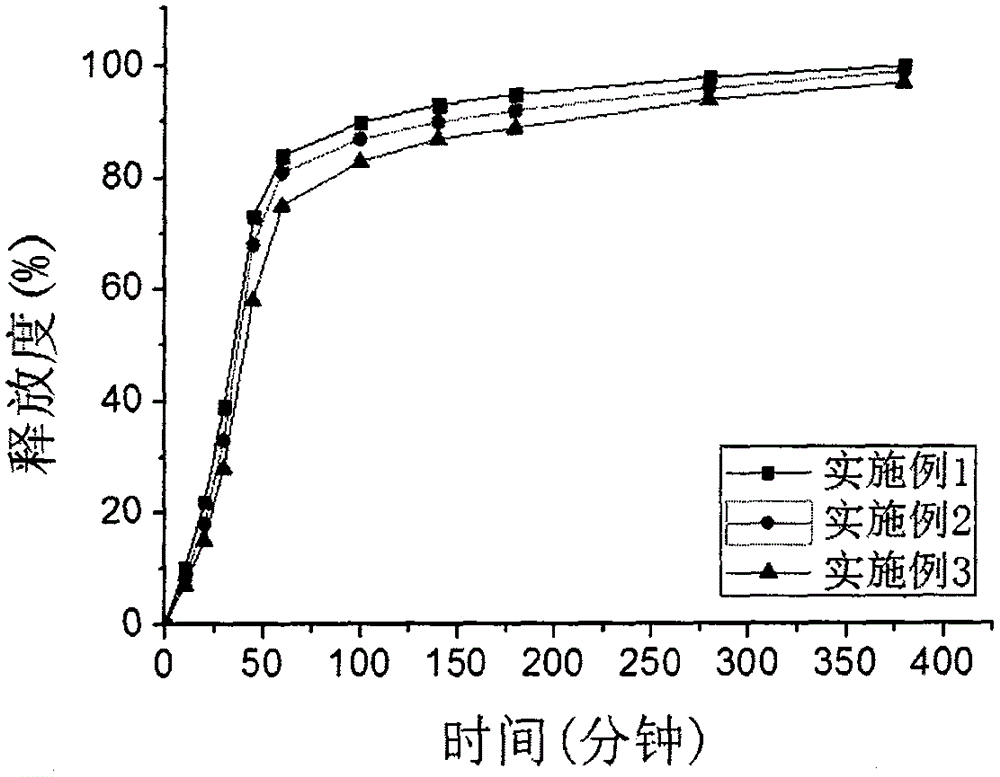
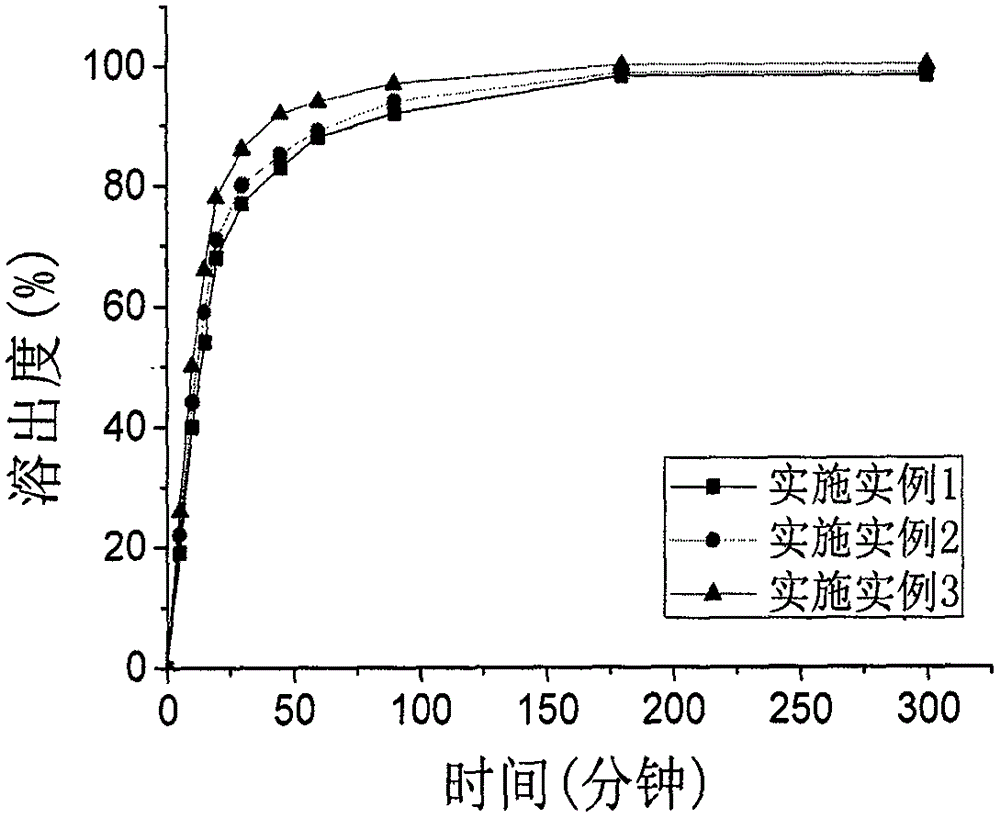
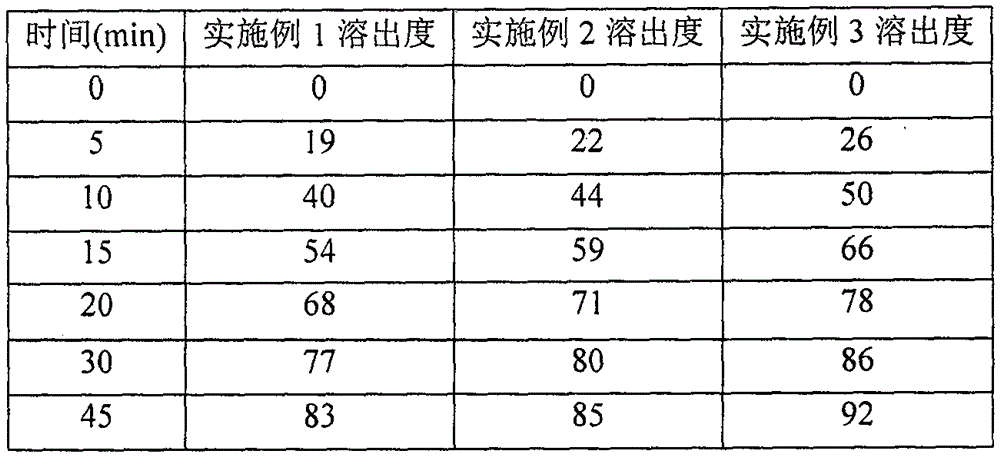
[0048] The capsules manufactured in each example were dissolved by rotating a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com