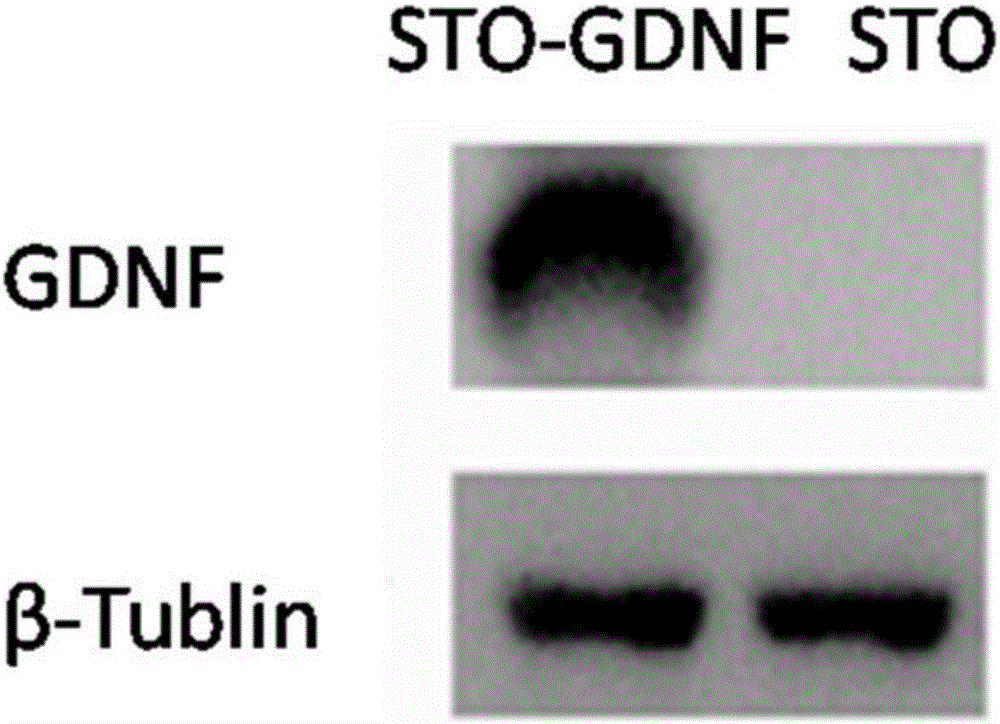
Spermatogonial stem cell culture system without adding recombinant growth factors
A spermatogonial stem cell and growth factor technology, applied in the field of spermatogonial stem cell culture, can solve the problems of high cost of culture, no support for long-term culture in vitro, easy cell differentiation, etc., and achieve clear medium composition, complete regeneration potential, and good repeatability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Spermatogonia stem cell (SSC) culture medium 500mL formula: basal medium (MEMα), bovine serum albumin (BSA) 0.6%, transferrin (Tf) 100μg / mL, unsaturated fatty acid (FFA) 15.2μeq / L , sodium selenite (Na 2 SeO 3 )6×10 - 8 M, 2-mercaptoethanol (2-ME) 100μM, insulin (InsuLin) 25μg / mL, 4-hydroxyethylpiperazineethanesulfonic acid (HEPESBUFFER) 10mM, putrescine (Putrescine) 120μM, penicillin (Penicillin) 50units / mL (Gibco15140122 (article number)), streptomycin (Streptomycin) 50μg / mL (Gibco15140122 (article number)), L-glutamine (L-glutamine) 2mM, 0.22μm filter filtration; Every milliliter described unsaturated fatty acid (FFA 100meq / L) includes: Linolenic acid 5.6mM, Oleic acid 13.4mM, Palmitoleic acid 2.8mM, Linoleic acid 35.6mM, Hexadecanoic acid (Palmitic acid) 31mM, octadecanoic acid (Stearicacid) 11.6mM, absolute ethanol as solvent.
[0023] 2. 500mL formula of culture medium used for STO cell line culture: basic high pond medium (DMEM): 455mL, serum (FBS): 35mL...
Embodiment 2
[0045] Compared with Example 1, this example only uses common STO cells as trophoblast cells.
[0046] 1. Spermatogonia stem cell (SSC) serum-free medium 500mL formula: basal medium (MEMα), bovine serum albumin (BSA) 0.6%, transferrin (Tf) 100μg / mL, unsaturated fatty acid (FFA) 15.2μeq / L, sodium selenite (Na 2 SeO 3 )6×10 -8 M, 2-mercaptoethanol (2-ME) 100μM, insulin (InsuLin) 25μg / mL, 4-hydroxyethylpiperazineethanesulfonic acid (HEPESBUFFER) 10mM, putrescine (Putrescine) 120μM, penicillin (Penicillin) 50units / mL (Gibco15140122 (article number)), streptomycin (Streptomycin) 50μg / mL (Gibco15140122 (article number)), L-glutamine (L-glutamine) 2mM, 0.22μm filter filtration; Every milliliter described unsaturated fatty acid (FFA 100meq / L) includes: Linolenic acid 5.6mM, Oleic acid 13.4mM, Palmitoleic acid 2.8mM, Linoleic acid 35.6mM, Hexadecanoic acid (Palmitic acid) 31mM, octadecanoic acid (Stearicacid) 11.6mM, absolute ethanol as solvent.
[0047] 2. 500mL formula of cult...
Embodiment 3
[0053] Compared with Example 1, this example only adds 1% serum (FBS) by volume to the spermatogonial stem cell culture medium.
[0054] 1. Spermatogonia stem cell (SSC) serum-containing culture medium 500mL formula: basal medium MEMα, bovine serum albumin 0.6%, transferrin 100 μg / mL, unsaturated fatty acid 15.2 μeq / L, sodium selenite 6×10 -8 M, 2-mercaptoethanol 100μM, insulin 25μg / mL, 4-hydroxyethylpiperazineethanesulfonic acid 10mM, putrescine 120μM, penicillin 50units / mL, streptomycin 50μg / mL, L-glutamine 2mM,, FBS (Fetal bovine serum): 5mL, filtered through a 0.22μm filter; the unsaturated fatty acids per milliliter include: linolenic acid 5.6mM, octadecenoic acid 13.4mM, palm oleic acid 2.8mM, linoleic acid 35.6mM, hexadecane Acid 31mM, octadecanoic acid 11.6mM, absolute ethanol as solvent.
[0055] 2. 500mL formula of culture medium used for STO cell line culture: basic high pond medium (DMEM): 455mL, serum (FBS): 35mL, penicillin-streptomycin (P&S): 5mL, L-glutamine (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com