Application of specific cellular pharmaceutical mixture in skin beauty
A technology of drugs and mesenchymal stem cells, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] In this example, the inventors screened the combination of mesenchymal stem cells from different sources with collagen or hyaluronic acid or calcium hydroxyapatite (microcrystalline porcelain) or polylactic acid or fat or platelet-rich plasma. Improve the effect of cell activity. In this embodiment, the cells to be improved are fibroblasts. Mesenchymal stem cells from different sources include: cord blood-derived, peripheral blood-derived, umbilical cord-derived, placenta-derived, Amniotic membrane-derived, bone marrow-derived, periosteum-derived, adipose-derived, tooth-derived, etc. The inventors found that compared with other sources of mesenchymal stem cells, umbilical cord-derived mesenchymal stem cells belong to medical waste, have lower cell immunogenicity, and have certain versatility. The obtained cells are of high quality and large quantity , The purity is also higher. Furthermore, in subsequent experiments, the inventors selected a pharmaceutical composition ...
Embodiment 2
[0055] In this example, the inventors observed and identified the morphology and phenotype of the selected umbilical cord mesenchymal stem cells. The results are described below:
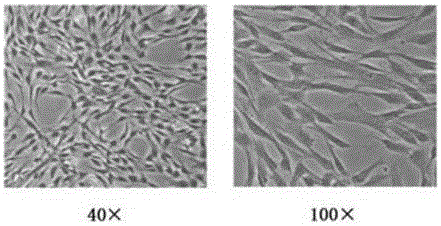
[0056] The morphology of umbilical cord mesenchymal stem cells showed that the cells adhered to the wall, showed spindle shape, and grew in sheets. Specific forms such as figure 1 shown. Umbilical cord mesenchymal stem cells in figure 1Under the growth state of umbilical cord mesenchymal stem cells, the proliferative ability of umbilical cord mesenchymal stem cells is strong, and the cytokines secreted including bFGF, VEGF, IGF, etc. stem cell.
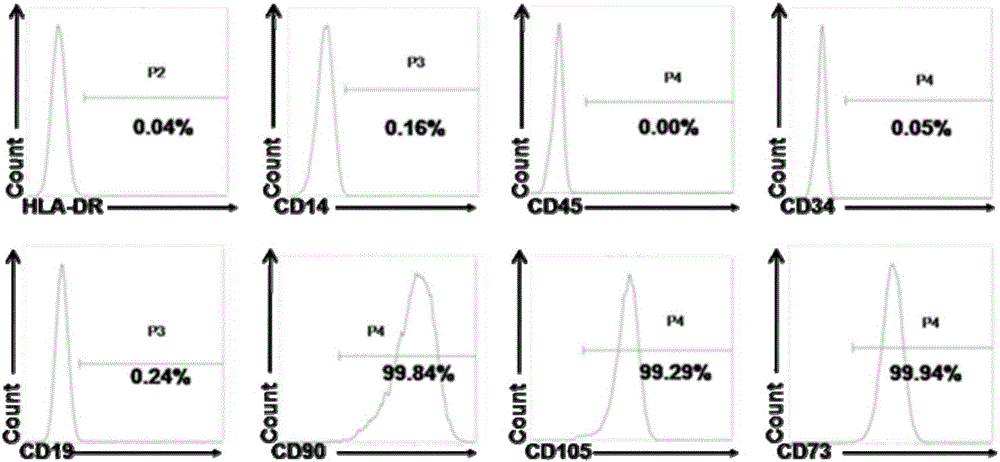
[0057] The inventor identified by flow cytometry that the selected umbilical cord mesenchymal stem cells express CD90 (positive cell rate: 99%), CD105 (positive cell rate: 99%), CD73 (positive cell rate: 99%), and do not express HLA -DR, CD14, CD19, CD34 and CD45, the results are as figure 2 shown.
Embodiment 3
[0059] In this example, the inventors further sorted the obtained umbilical cord mesenchymal stem cells by cell surface markers. The inventors found that according to NG-2, Cx43, DLK1, PDGFR-α (CD140a), PDGFR-β ( CD140b), CD13, CD44, CD105, CD133, CD146, CD248, Flt-1 and KDR (CD309) at least one of the cell surface markers after sorting, the resulting mesenchymal stem cells on cell viability, vegetative cells, cell Further vascularization has a clear promoting effect.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com