Solid-phase synthesis method of Sermaglutide
A technology for semaglutide and solid-phase synthesis, which is applied in the preparation methods of peptides, chemical instruments and methods, peptides, etc., can solve the problems of unfavorable large-scale production of semaglutide, high production cost, long synthesis cycle and the like, Achieve considerable economical value, improve condensation efficiency, and improve the effect of yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 The transformation of lysine raw material
[0025] (1) Connection of side chain amino acids
[0026] Add 1 / 3-1 / 2 reactor volume of 20% piperidine / DMF deprotection solution to the drained reactor, place on a 30r / min decolorizing shaker and shake for 20min to remove the Fmoc side of lysine Chain protection group, according to the solid-phase amino acid condensation method, sequentially couple 2-(2-(2-aminoethoxy)ethoxy)acetic acid, 2-(2-(2-aminoethyl) on the side chain of lysine Oxy)ethoxy)acetic acid, Glu and octadecanedioic acid, after successful condensation, wash with DMF 5 times, and drain.
[0027] (2) Remove Dde protecting group
[0028] According to the configuration of hydrazine hydrate: DMF = 1:15 to remove the solution, eluted with a mixed solution of three times the volume of the resin each time, and washed 3 times with DMF after shaking for 5 minutes. After the third time, the hydrazine hydrate solution was removed. Second, the modified lysine...
Embodiment 2
[0029] Example 2 Synthesis of Semaglutide-2-Cl-Resin
[0030] 1.2 Swelling of Cl-Resin
[0031] Weigh 1g of 2-Cl-Resin with a substitution degree of 0.64mmol / g, add it to the polypeptide synthesis reactor from the open end, take the DCM reagent and add it to the reactor, so that the resin is completely immersed in the DCM solvent and fully contacted with the solvent , swelling for 0.5h.
[0032] 2. Synthesis of Semaglutide-2-Cl-Resin
[0033] Semaglutide-2-Cl-Resin is:
[0034] H-Aib-EGTFTSDVSSYLEGQAAK (Octadecanedioic-Glu-PEG2-PEG2) EFIAWLVRG RG-2-Cl-Resin
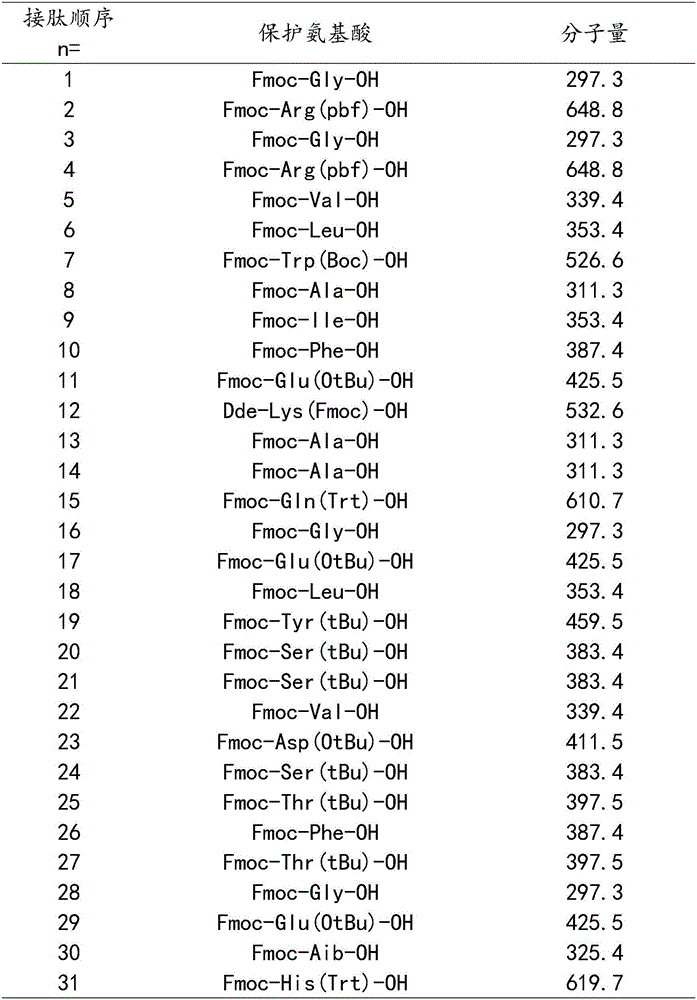
[0035] The protected amino acids used in this example are calculated from the resin, and the protected amino acids and molecular weights corresponding to the 1-31st amino acid are shown in the following table:
[0036]
[0037] Some commonly used abbreviations in the present invention have the following meanings:
[0038] Fmoc: fluorenylmethoxycarbonyl
[0039] DMF: N,N-Dimethylformamide
[0040] DCM: dichlorome...
Embodiment 3
[0060] Example 3 The cleavage and sedimentation of crude peptide products of semaglutide
[0061] (1) Configure cutting reagents
[0062] The 100ml formula is: 87.5mlTFA+5ml thioanisole+5ml water+2.5mlEDT+5g phenol, placed in a brown reagent bottle. It is now equipped for use, and the preparation amount is generally 1g resin plus 10ml cutting reagent.
[0063] (2) Cutting of semaglutide-2-Cl-Resin
[0064] Add the cleavage reagent to the drained reactor, place it on a decolorizing shaker and shake it for 1 hour at a speed of 20 r / min. After cutting, add about 35ml of glacial ether to a 50ml centrifuge tube, filter the solution in the reactor into glacial ether through a sand core, cover the cap of the centrifuge tube, shake the centrifuge tube up and down, and mix well. Put the centrifuge tube into the centrifuge, 3000r / min, 3min, centrifuge to discard the supernatant, repeat the operation 3 times to obtain the crude peptide product of semaglutide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com