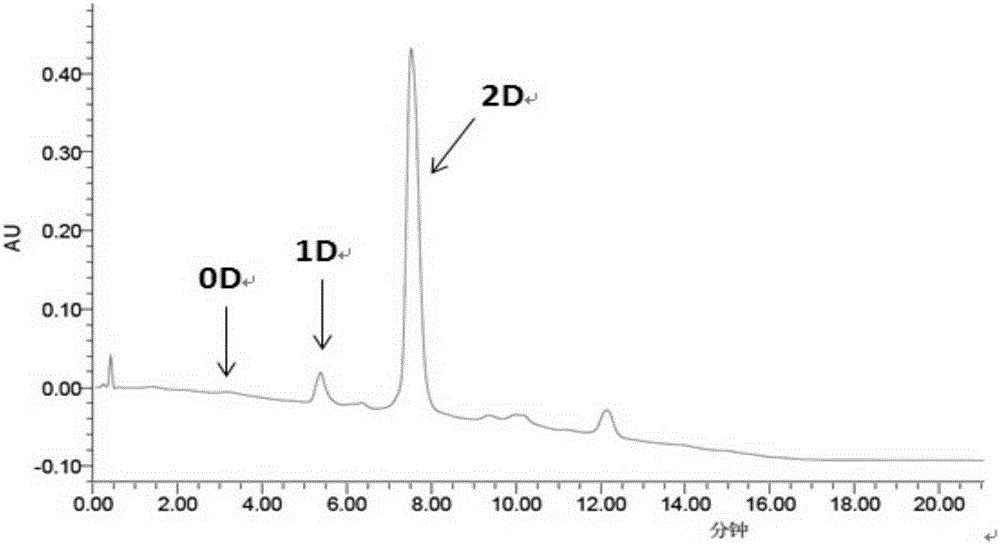
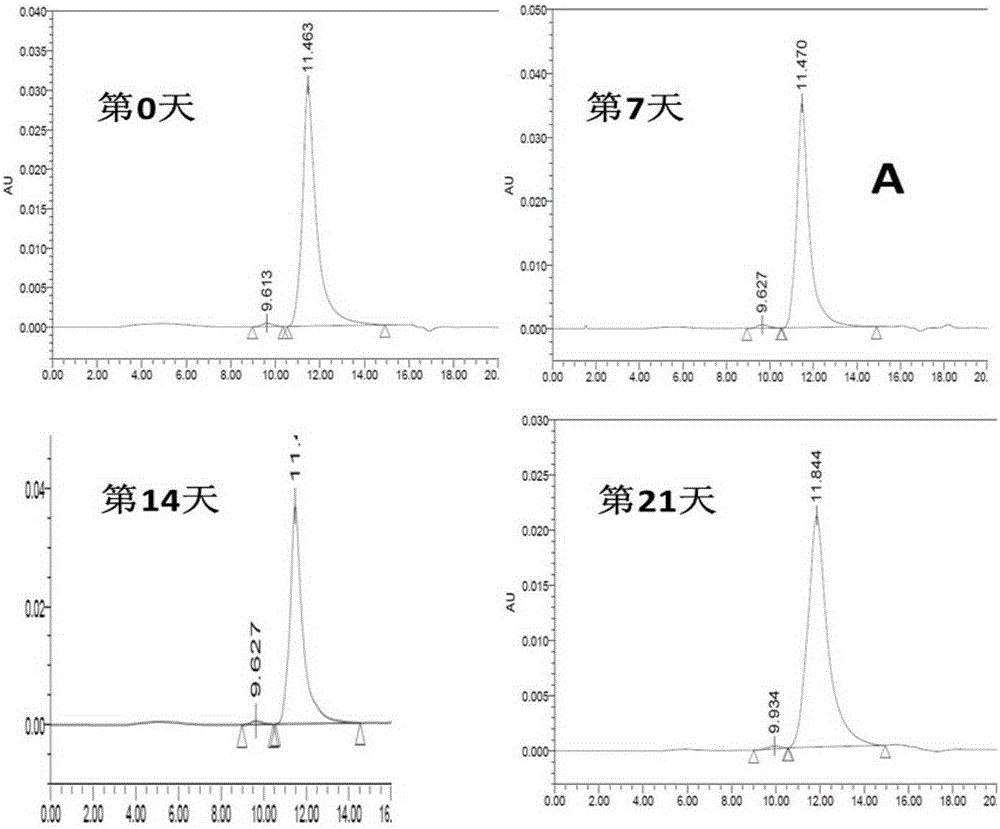
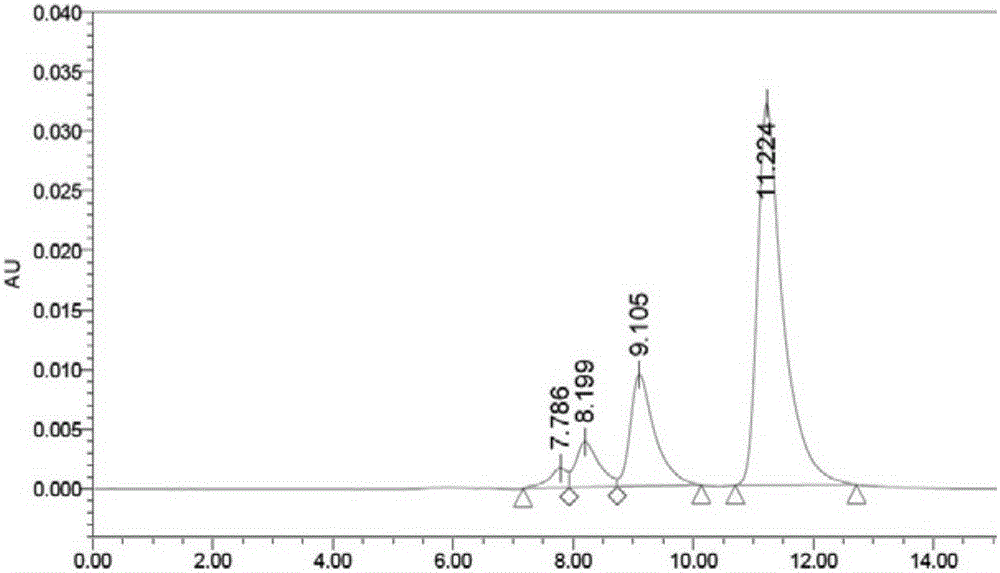
Cysteine-modified antibody drug conjugate
A technology of cysteine and conjugates, applied in the field of compounds and their preparation, can solve the problems of poor drug uniformity, toxic and side effects of patients, and limitation of clinical dosage, and achieve the effect of less side effects and good drug uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The synthesis of embodiment 1 mc
[0030]
[0031] 3.9 g (0.03 mol) of 6-aminocaproic acid and 3.5 g (0.036 mol) of maleic anhydride 1.2 eq were added to 30 ml of glacial acetic acid. The reaction solution was stirred at 120°C for 4-6 hours. After the reaction was completed, the heating was stopped, and it was naturally cooled to room temperature. Concentrate under reduced pressure at 60°C to remove most of the acetic acid. The resulting brown-yellow viscous liquid was poured into water, and 20ml×3 ethyl acetate was added for extraction, and the organic layers were combined. The organic layer was washed with water and saturated brine successively, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain a brownish-yellow oil, which was stirred with 50ml of water, and an off-white solid was precipitated, filtered, and the target product 5.08 was dried under reduced pressure at 50°C g, yield 80%. mp: 89-92°C....
Embodiment 2
[0032] Synthesis of Example 2 Mc-OSu
[0033]
[0034]Add 4.7g (22mmol) MC and 25g (22mmol) HOSu to 50ml acetonitrile under nitrogen protection. Another 4.5 g (22 mmol) of DCC was dissolved in 25 ml of acetonitrile, and the internal temperature was kept at about 0° C., and slowly dropped into the reaction solution. The reaction solution was reacted at 0°C for 2 hours, and then reacted overnight at room temperature. After filtering, the filter cake was washed with acetonitrile 10ml×3, and the filtrate was concentrated to dryness under reduced pressure. The obtained oil was dried under reduced pressure at room temperature for 6 h to obtain 6.4 g of a light brown solid with a yield of 95%. (Directly put into the next reaction without purification) m / z: 309.2[M+H]+. 1HNMR (400Mz, CDCl3): 1~2(m, 6H, CCH2CH2CH2C), 2.68(t, 2H, CH2CO), 2.95(s, 4H, COCH2CH2CO), 3.68(t, 2H, CH2N), 6.81(s, 2H ,CH=CH).
Embodiment 3
[0035] Synthesis of Example 3 Fmoc-Val-OSu
[0036]
[0037] 10 g of Fmoc-Val and 3.4 g of HOSu were added to 100 ml of THF. Another 6 g of DCC was dissolved in 50 ml of acetonitrile, and the internal temperature was kept at about 0°C, and slowly dropped into the reaction solution. The reaction solution was stirred at room temperature for 24 hours. After filtration, the filter cake was washed with THF, and the filtrate was concentrated under reduced pressure to obtain a transparent oil. The oil was directly used for the next reaction without purification. m / z: 437.4[M+H]+
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com