Synthesis method of levo-amino compound
A synthetic method and technology of L-amine group, which is applied in the field of preparation of optically pure L-chiral amine compounds, can solve problems such as not being seen, and achieve the effects of good product yield, easy availability of raw materials, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
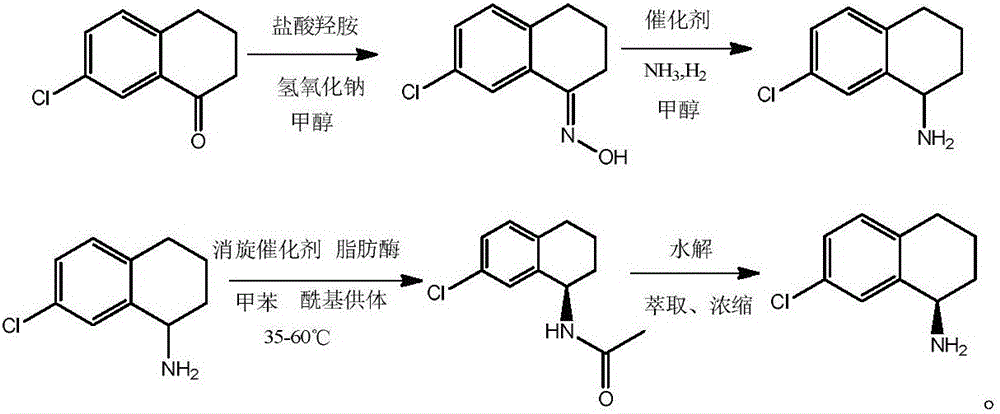
[0007] 1. Synthesis of 7-Chloro-1 Tetralone Oxime
[0008] In the three-necked flask, add 500ml of methanol as a solvent, add 90g of raw materials 7-chloro-1-tetralone, 38.2g of hydroxylamine hydrochloride, and dropwise add a concentration of 40% sodium hydroxide solution in batches under the condition of stirring at room temperature until the pH of the system is Weakly alkaline, continue to stir and react for 2 hours after adding, stop the reaction when the point of raw material 7-chloro-1 tetralone disappears by pointing the plate; under stirring conditions, add 2000ml of water to the system, and a large amount of white solids precipitate out; After suction filtration, the resulting filter cake was washed with water until it became neutral, and then dried for later use. In this step, 95.9 g of 7-chloro-1-tetralone oxime was obtained, with a yield of 98.4%.
[0009] 2) Reductive amination of 7-chloro-1 tetralone oxime
Embodiment 2
[0016] 1) Synthesis of 7-Chloro-1 Tetralone Oxime
[0017] In the three-necked flask, add 1000ml of methanol as a solvent, add 180g of raw materials 7-chloro-1-tetralone, 77g of hydroxylamine hydrochloride, and add a concentration of 40% sodium hydroxide solution in batches under the condition of stirring at room temperature until the pH of the system is weak. Alkaline, continue to stir and react for 2.5 hours after adding, stop the reaction when the point of raw material 7-chloro-1 tetralone disappears by pointing the plate; under stirring conditions, add 4000ml of water to the system, a large amount of white solids are precipitated; After filtering, the resulting filter cake was washed with water until neutral, and dried for later use. This step gave 193.2 g of 7-chloro-1 tetralone oxime, with a yield of 99.1%.
[0018] 2) Reductive amination of 7-chloro-1 tetralone oxime
[0019] In the autoclave, add 600ml of anhydrous methanol as a solvent, 193.2g of 7-chloro-1 tetralone...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com