Stabilization of cytochrome P450 reductase
A technology of cytochrome and reductase, applied in the direction of oxidoreductase, enzyme, bacteria, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0097] 1. A recombinant host cell capable of expressing cytochrome P450 enzymes (CYP) and cytochrome P450 reductase (CPR) and capable of overexpressing the amino acid sequence comprising SEQ ID NO: 2 or having at least 50% thereof Ice2p of sequences of sequence identity.
[0098] 2. The recombinant host cell according to embodiment 1, wherein the CYP is the tofu spirodiene oxygenase CYP71D55 (HPO) from Hyoscymaus muticus, optionally in which mutations V482I and A484I have been carried out, from Mentha piperita (-)-limonene-3-hydroxylase (PM17) or human cytochrome P450 2D6 (CYP2D6).
[0099] 3. The recombinant host cell according to embodiment 1 or 2, wherein the CRP is cytochrome P450 reductase from A. thaliana or human reductase.
[0100] 4. The recombinant host cell according to any one of the preceding embodiments, which is capable of producing a compound of interest.
[0101] 5. The recombinant host cell according to embodiment 4, wherein the compound of interest is a st...
Embodiment 1
[0173] Example 1: Screening for effectors of CYP / CPR function in recombinant S. cerevisiae strains
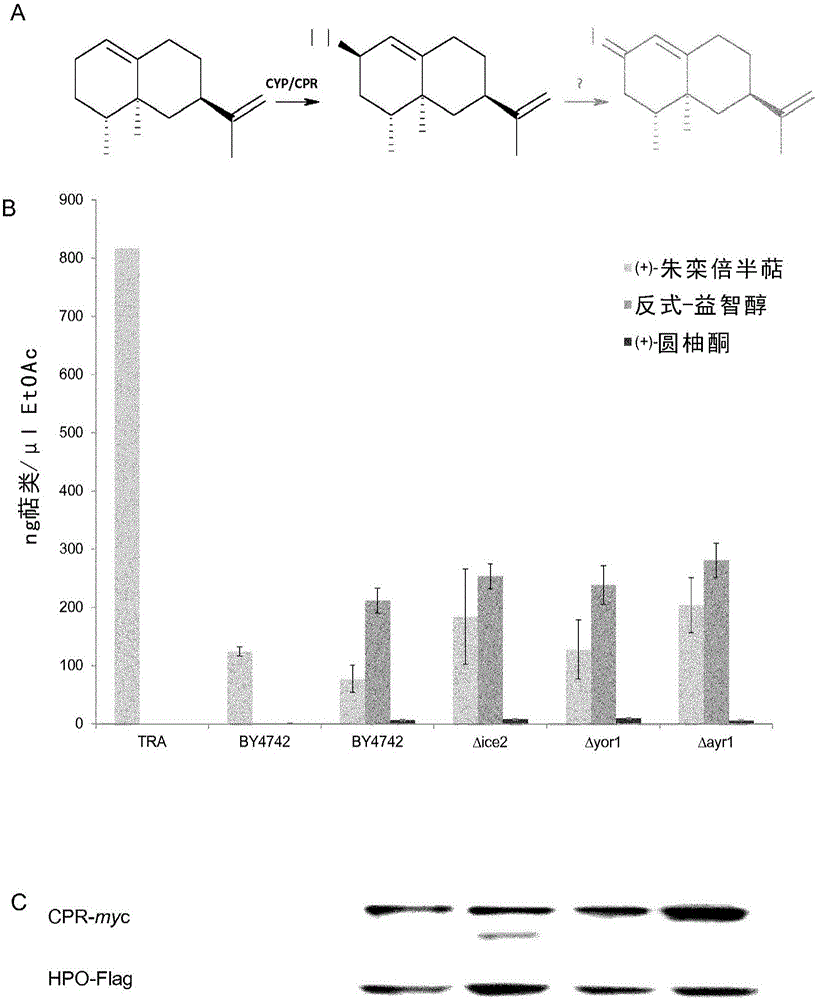
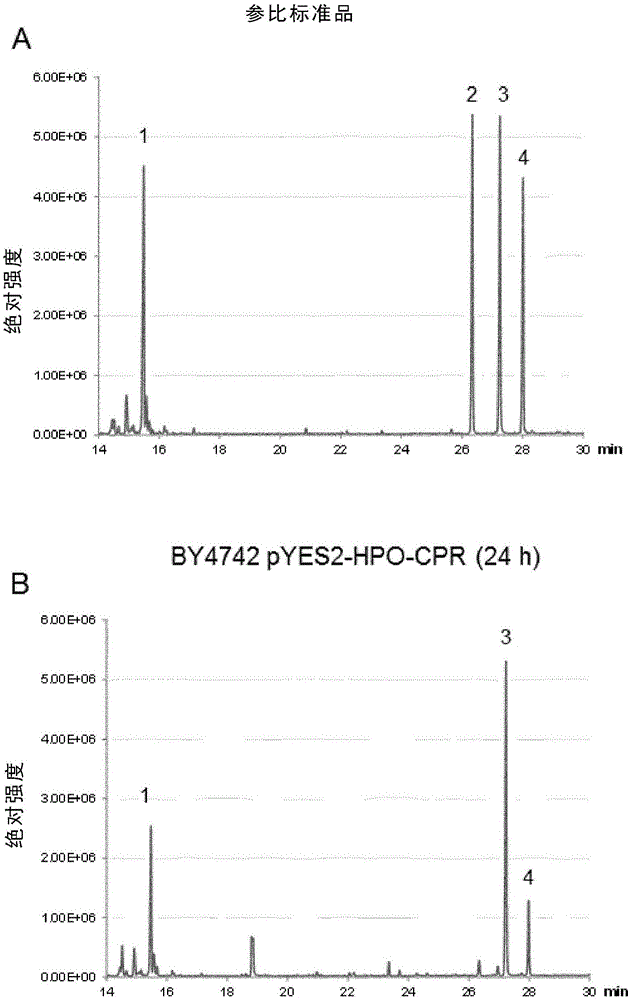
[0174] (+)-valencene is a by-product of orange juice production and is of low commercial importance. However, many attempts have been made to convert (+)-valencene into the attractive flavor and aroma compound (+)-narone by various biocatalytic pathways (by (Fraatz et al., 2009) review). As highly stereoselective and regioselective catalysts, soluble and membrane-attached cytochrome P450 enzymes (CYPs) have been tested for this reaction (Cankar et al., 2011; Gavira et al., 2013; Girhard et al., 2009; Takahashi et al., 2007). There are solid data emphasizing that CYPs indeed hydroxylate (+)-valencene to produce nootropes ( figure 1 ), but the oxidation of the latter compound to narone is carried out by the endogenous activity of S. cerevisiae (Gavira et al., 2013) or Pichia pastoris (Wriessnegger et al., revised manuscript). An important feature of CYPs is their requiremen...
Embodiment 2
[0175] Example 2: Overexpression of ICE2 in S. cerevisiae W303
[0176] Among the various S. cerevisiae host strains for CYP / CPR expression and CYP-mediated biotransformation, especially the W303 strain proved to be the most suitable host (Loeper et al., 1998; Pompon et al., 1996; Truan et al. et al., 1993; Urban et al., 1994). Many standard laboratory strains of S. cerevisiae, but not the W303 strain, carry deletions in the HAP1 gene. Haplp is a transcription factor responsible for regulating genes that control intracellular heme abundance in response to changes in available oxygen levels (B.S.J. Davies and Jasper Rine, 2006; Ihrig et al., 2010). The Δhap1 genotype strains are said to be less favorable for CYP expression and function because these strains are strongly dependent on optimal heme and oxygen supply. To test host strain effects, HPO / CPR-mediated (+)-valencene conversion of resting cells was performed in parallel in BY4742 and W303 yeast strains with pYES-HPO-C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com