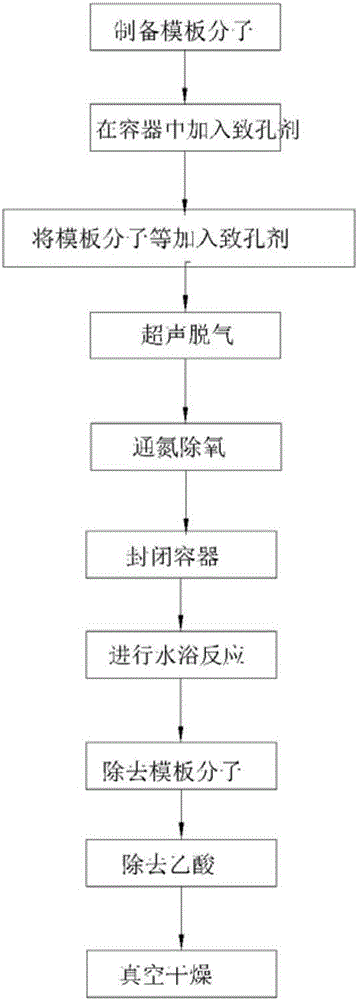
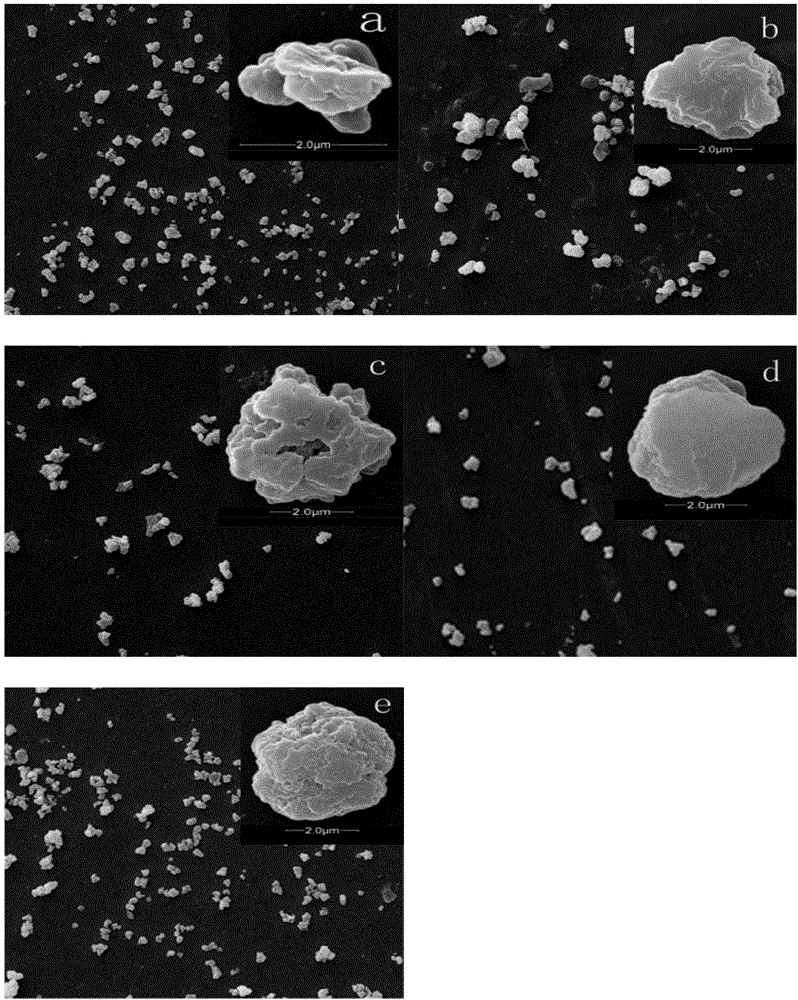
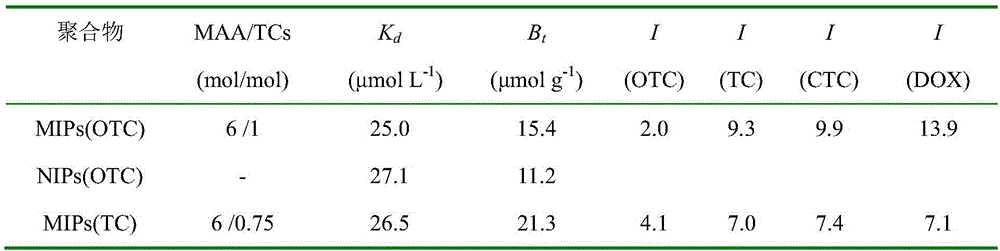
High selectivity tetracycline antibiotics molecularly imprinted polymer preparation method and application
A technology of molecular imprinting and tetracyclines, applied in chemical instruments and methods, material separation, analysis of materials, etc., can solve problems that cannot meet application needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The following reagents will be used in this example: Tetracycline (TC), Oxytetracycline (OTC), Chlortetracycline (CTC), Doxycycline (DOX), Methacrylic acid (MAA), Trimethylol Trimethylolpropane trimethylacrylate (TRIM), each of the above reagents were purchased from Sigma (USA). In this example, methanol (chromatographically pure), acetonitrile (analytical pure), and 2,2-azobisisobutyronitrile (AIBN) were also used. These reagents were purchased from Tianjin Komiou Reagent Company. Among them, MAA needs to be distilled away from light and reduced pressure under the protection of nitrogen to remove the polymerization inhibitor and store in the dark. AIBN needs to be dissolved in methanol, filtered, cooled and crystallized and dried for later use.
[0048] McIlvaine buffer is prepared as follows: accurately weigh out 11.8g of citric acid, 13.72g of disodium hydrogen phosphate and 33.62g of disodium edetate and dissolve in ultrapure water, and place the volume in a brown vol...
Embodiment 2
[0068] In this example, based on the preparation of molecularly imprinted polymers for four tetracycline antibiotics, a dual-template molecularly imprinted polymer was prepared. At present, most of the MIPs that specifically recognize tetracycline antibiotics are prepared using OTC and TC as template molecules, and there are no reports in the literature that CTC and OTC are used as template molecules to prepare MIPs.
[0069] The preparation method of this embodiment is as follows:
[0070] (1) Prepare the target antibiotic template molecule. In this embodiment, two template molecules of oxytetracycline (OTC) and chlortetracycline (CTC) are used.
[0071] (2) Add 60 mL of porogen into a 100 mL round-bottom glass bottle, the porogen is a mixed solution of methanol and acetonitrile;
[0072] (3) Combine 1:1 OTC and CTC template molecules, methacrylic acid (MAA, 6mmol), crosslinking agent (trimethylolpropane trimethacrylate TRIM, 3mL), initiator (azobisiso Butyronitrile AIBN, 30mg) diss...
Embodiment 3
[0086] In this embodiment, dual-template MIPs are still prepared, but in this embodiment, different solvents are used to dissolve the obtained molecularly imprinted polymers and identify antibiotics.
[0087] The solvents used were water, methanol, acetonitrile, methanol / acetonitrile mixture (1 / 1, V / V)).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com