Method for sulfonated polyaniline catalytic synthesis of 5-hydroxymethylfurfural from carbohydrates
A technology of sulfonated polyaniline catalytic carbon and hydroxymethylfurfural, applied in organic compound/hydride/coordination complex catalysts, chemical instruments and methods, catalyst activation/preparation, etc., can solve the problem of poor catalyst stability and HMF selection Low stability, difficult to separate and purify, etc., to achieve the effect of low cost, easy to separate and purify, and not easy to dissolve
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] 0.16g (0.9mmol) of m-aminobenzenesulfonic acid and 0.26g (2.7mmol) of freshly distilled aniline were prepared into a 40mL monomer deionized aqueous solution (the molar ratio of m-aminobenzenesulfonic acid to aniline was 1:3), 3.00 g ammonium persulfate was prepared into 20mL deionized water solution, the molar ratio of ammonium persulfate to monomer was 11:3, pour the ammonium persulfate solution into the monomer solution at one time, control the temperature of the solution to 5℃, and let it stand for 24 hours The solid was filtered off with suction, washed with deionized water until the pH of the filtrate was neutral, and the solid was vacuum dried at 100° C. for 12 hours to obtain a catalyst.
[0014] Weigh 15 mg of the above catalyst in a thick-walled glass pressure tube, and then add a magnetic stir bar, 45.0 mg fructose and 1 mL deionized water-1,4-dioxane solvent (deionized water and 1 , 4-Dioxane volume ratio is 1:19), after stirring evenly, pass N 2 Exhaust the air...
Embodiment 2-8
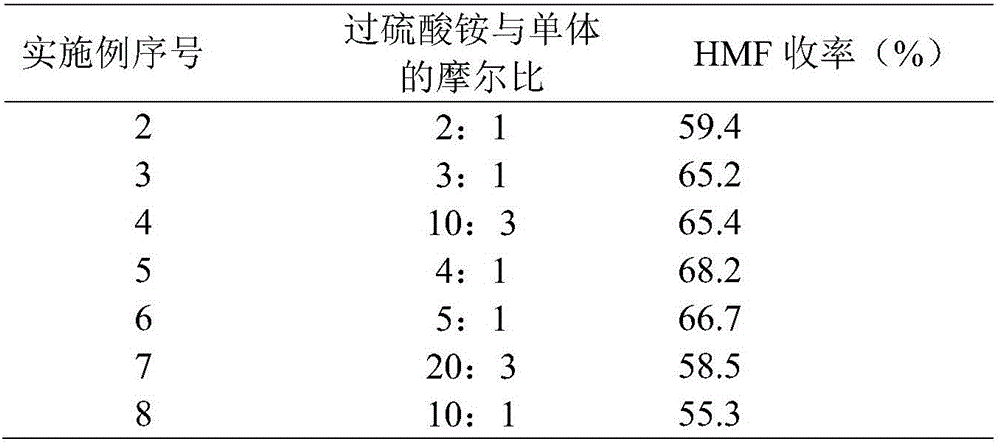
[0016] The catalyst was prepared according to the method of Example 1, and the molar ratio of ammonium persulfate to monomer was changed to 2:1, 3:1, 10:3, 4:1, 5:1, 20:3, and 10:1 to prepare The obtained catalyst was used in the dehydration reaction of fructose, and the reaction results are listed in Table 1.
[0017] Table 1.
[0018]
Embodiment 9-15
[0020] The catalyst was prepared according to the method of Example 1. The prepared catalyst was used for the dehydration reaction of fructose, and the volume ratio of deionized water to 1,4-dioxane was changed to 0:1, 1:99, 1:39, 1:12.3, 1:9, 1:5.7 and 1:4, the reaction results are listed in Table 2.
[0021] Table 2.
[0022]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com