Abamectin dispersible tablets and preparation method thereof
A technology of abamectin and dispersible tablets, applied in the field of medicine, can solve the problems of low bioavailability, slow disintegration and the like, and achieve the effects of high release, good application prospect and excellent disintegration performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
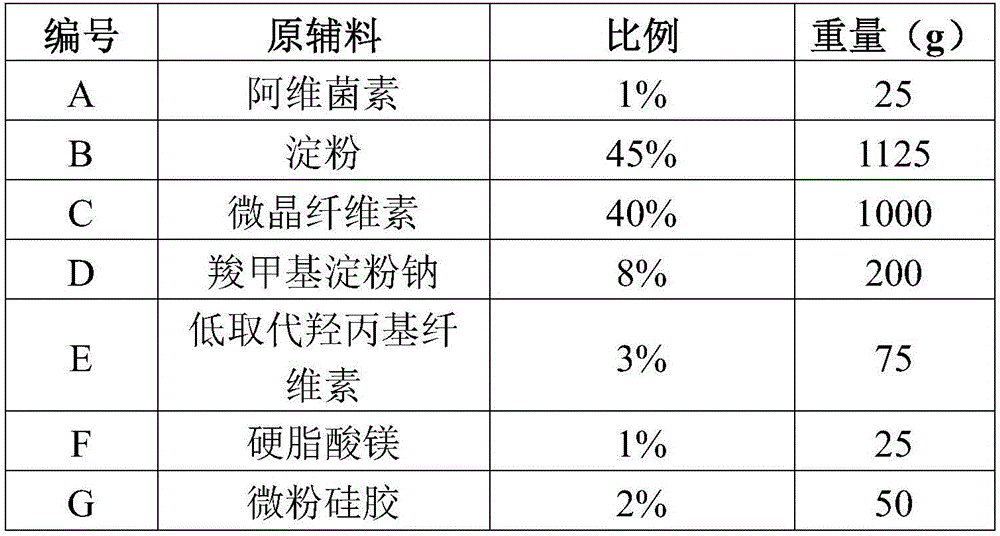
[0019] Example 1 Preparation method of abamectin dispersible tablets of the present invention (prescription is based on 1000 tablets)
[0020]
[0021] Preparation:
[0022] (1) Ultrafine pulverization of A at low temperature (-20°C) for 30 minutes to obtain ultrafine powder with a particle size of less than 50μm, and B-G respectively through 80 mesh sieve for use.
[0023] (2) Mix AE for 30 minutes to obtain the mixture, add 20% ethanol to make soft material, pass through a 20-mesh sieve to granulate, and dry at 50°C for more than 12 hours to control the water content of the granules below 3%. Afterwards, add magnesium stearate and micro-powdered silica gel and mix for 30 minutes, press tablets, and get ready.
Embodiment 2
[0024] Example 2 Preparation method of abamectin dispersible tablets of the present invention (prescription is based on 1000 tablets)
[0025] Numbering Raw materials proportion Weight (g) A Avermectin1%25 B starch50%1250 C Microcrystalline cellulose35%875 D Sodium Carboxymethyl Starch8%200 E Low-substituted hydroxypropyl cellulose3%75 F Magnesium stearate2%50 G Micronized silica gel1%25
[0026] Preparation:
[0027] (1) Ultrafine pulverization of A at low temperature (-20°C) for 30 minutes to obtain ultrafine powder with a particle size of less than 50μm, and B-G respectively through 80 mesh sieve for use.
[0028] (2) Mix AE for 30 minutes to obtain the mixture, add 20% ethanol to make soft material, pass through a 20-mesh sieve to granulate, and dry at 50°C for more than 12 hours to control the moisture of the granules below 3%, and then pass through a 20-mesh sieve. Afterwards, add magnesium stearate and micro-powdered silica gel and mix for 30 minutes, press tablets, and get...
Embodiment 3
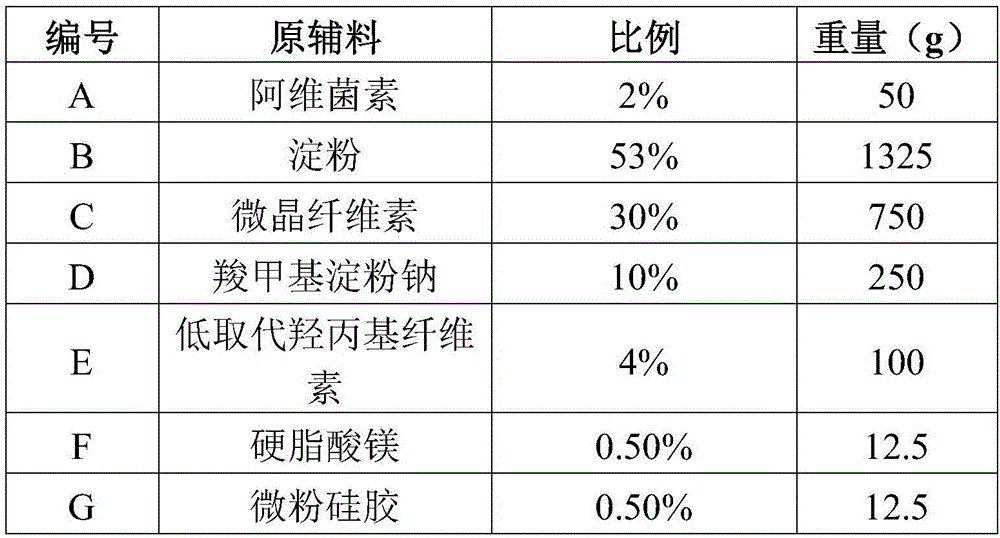
[0029] Example 3 Preparation method of abamectin dispersible tablets of the present invention (prescription is based on 1000 tablets)
[0030]
[0031] Preparation:
[0032] (1) Ultrafine pulverization of A under low temperature conditions (-20°C) for 30 minutes to obtain ultrafine powder with a particle size of less than 50μm, and B-G respectively through 80 mesh sieve for use.
[0033] (2) Mix AE for 30 minutes to obtain the mixture, add 20% ethanol to make soft material, pass through a 20-mesh sieve to granulate, and dry at 50°C for more than 12 hours to control the moisture content of the granules below 3%. Afterwards, add magnesium stearate and micro-powdered silica gel and mix for 30 minutes, press tablets, and get ready.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com