Method for inducing transdifferentiation of fibroblasts to nerve cells
A technology of fibroblasts and nerve cells, applied in the biological field, can solve the problems of efficiency to be improved, hidden dangers of system security, and high technical barriers.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
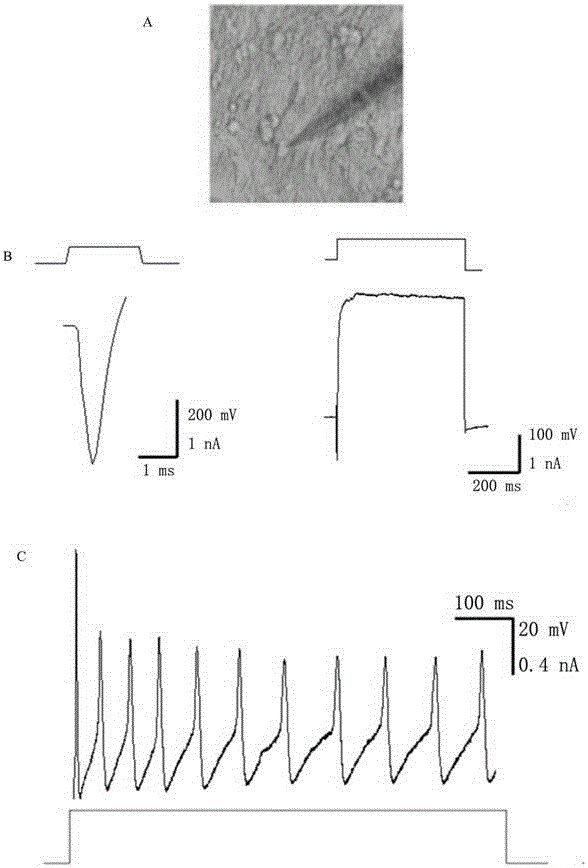
Image
Examples
Embodiment 1
[0028] (1) The preparation steps of mouse embryonic fibroblasts (MEFs) are as follows:
[0029] 1) Pretreatment of the culture vessel: Cover the bottom wall of the culture vessel with 0.2% gelatin, leave it at room temperature for 30 minutes, suck out the 0.2% gelatin, and set it aside at room temperature.
[0030] 2) Mice (wild-type mice or transgenic mice carrying GFAP-GFP reporter system) were injected with about 0.5 mL of avertin for anesthesia at 13.5 days of pregnancy, and the mice were killed by neck breaking, and immersed in 75% ethanol Disinfect for 5 minutes.
[0031] 3) Wipe the abdomen with 75% ethanol, cut the skin and pull the skin back to expose the abdominal wall. Cut the abdominal wall to expose the uterus. Move the uterus to a 100mm dish and wash it three times with 10mL PBS.
[0032] 4) Cut open the embryo sac with scissors, and move the embryos to a petri dish.
[0033] 5) Carefully remove the head and viscera of the embryo, transfer the torso of the em...
Embodiment 2
[0042] In order to explore the potential role of small molecules in changing cell fate, more than 10 small molecule compounds and their combinations that have been proven to have an impact on reprogramming were systematically analyzed and screened, including
[0043] HDAC inhibitors: NaB(N), VPA(V), TSA(A);
[0044] DNMT inhibitors: RG108(R), 5-AZA(5);
[0045] G9a inhibitor: BIX-01294(B);
[0046] Ezh2 inhibitors: DZNep (D), GSK126 (G);
[0047] LSD1 inhibitor: phencypromine (T);
[0048] AC inhibitor: Forskolin (F);
[0049] GSK3 inhibitor: CHIR99021(C);
[0050] MEK inhibitor: PD032590(P);
[0051] ALK5 inhibitors: A-83-01(8), E616452(6), SB431542(S).
[0052] The concentration of each small molecule compound is shown in Table 1.
[0053] Table 1
[0054] small molecule compound Concentration (μM) VPA(V) 500 NaB(N) 20 RG108(R), Forskolin(F), CHIR99021(C) 10 Phenylcypromine(T), E616452(6), SB431542(S) 5 5-AZA(5) 4 PD032590...
Embodiment 3
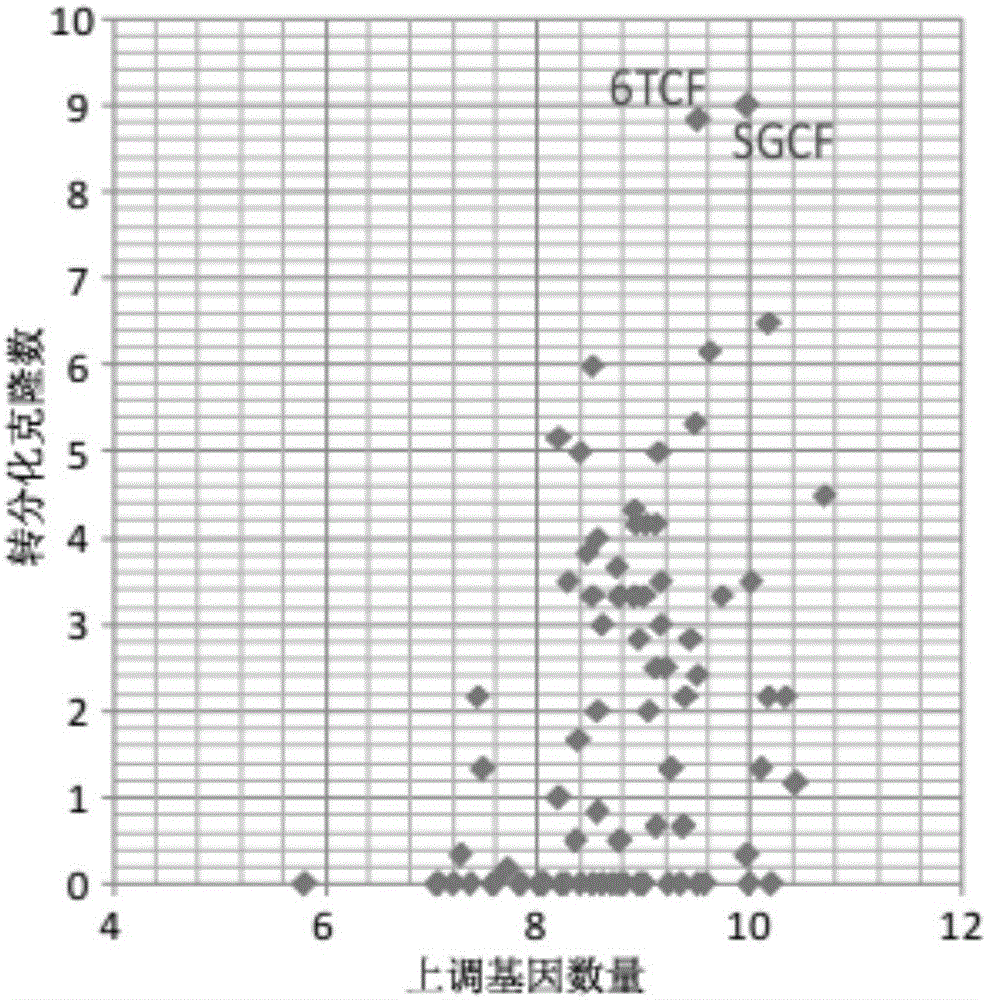
[0060] The TTFs cells prepared in Example 1 were inoculated on a 6-well plate for cell culture (20,000 cells per well), and different small molecule combinations were added to the medium from the second day, and then the medium was changed every four days. After 16 days of detection, the screening effect of different small molecule combinations was determined by counting the number of clones formed and qPCR analysis. Such as Figure 5 As shown, small molecules combined with 6TCF to treat TTFs can also obtain a-Actinin-positive cardiomyocyte-like cells and Tuj1-positive neuron-like cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com