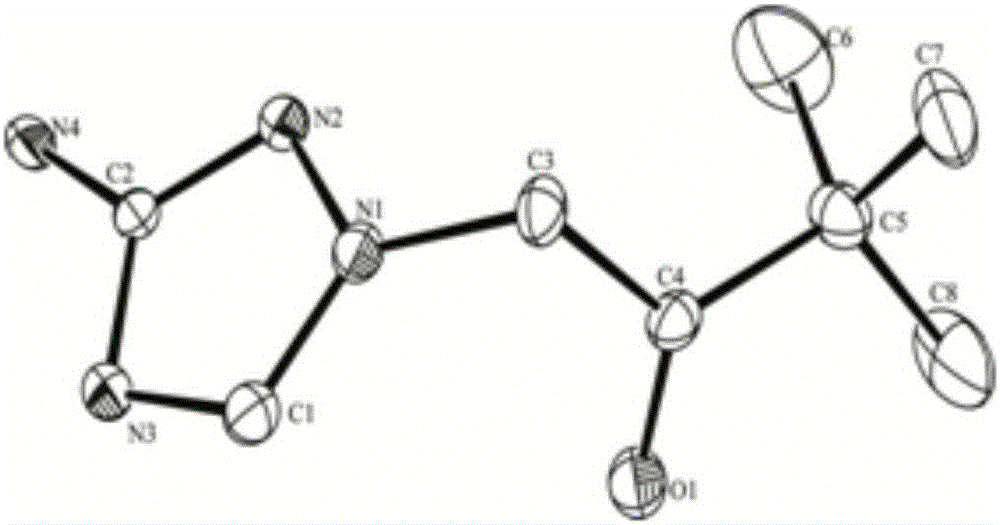
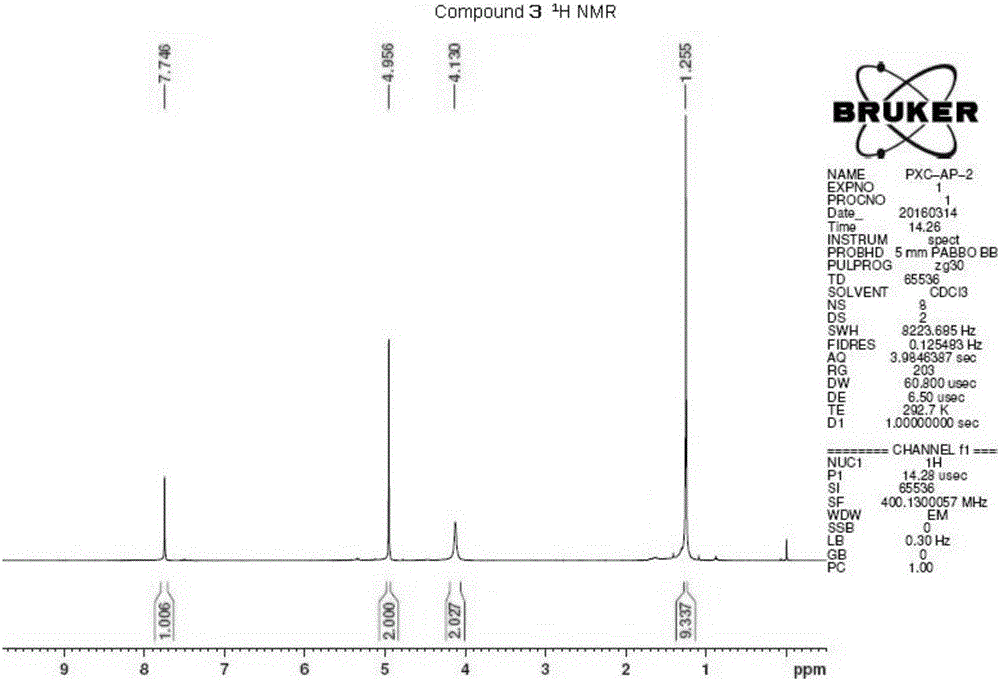
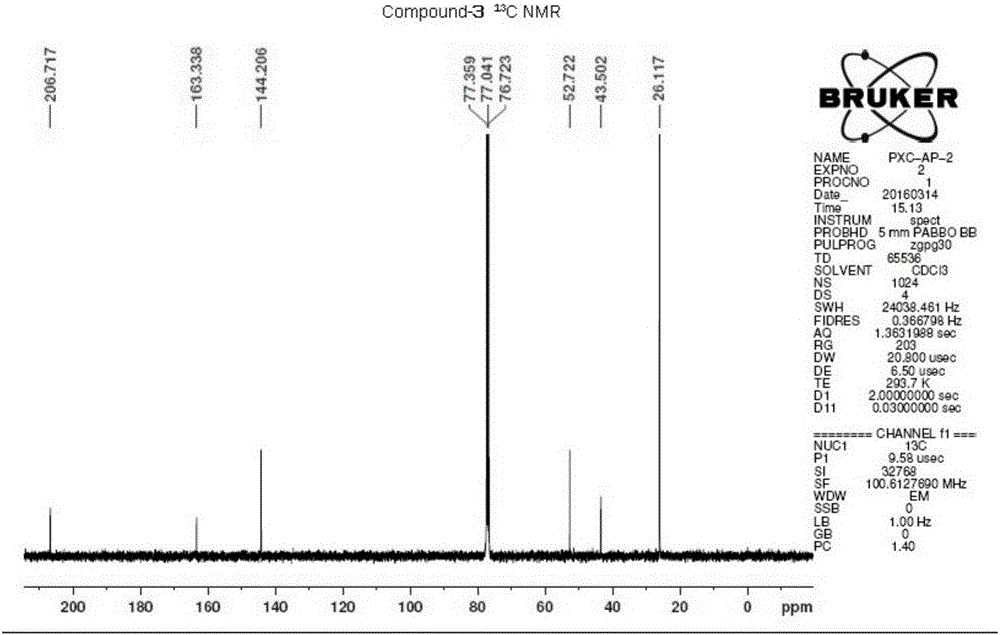
1-(3-amino-1, 2, 4-triazole)-base-3,3-dimethyl-2-butanone and application thereof
A dimethyl and amino technology, which is applied in the field of plant growth regulators, can solve the problems of plant dwarfing and other problems, and achieve the effects of good regulation, simple synthesis, and significant activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] This example illustrates the preparation of 1-(3-amino-1,2,4-triazol)-yl-3,3-dimethyl-2-butanone.
[0037] 0.12mmol of 3-methyl-1,2,4-triazole was mixed with an equivalent amount of pyridine, and pyridine was used as a catalyst and an acid-binding agent; 70mL of acetonitrile was added as a reaction solvent; 0.099mmol of 1-tert-butyl-2 - Chloro-ethanone and 20ml of acetonitrile are miscible and diluted, and the whole is added to the above reaction system; microwave radiation is 5min, and the radiation power is 100W; after the reaction is completed, cool to room temperature, concentrate under reduced pressure to remove the acetonitrile solvent, dissolve the product in ethanol, and filter , and wash the solid 3 times with ethanol, each time the amount of ethanol is not more than 20mL; the filtrate is combined and concentrated to about 5mL; the concentrated product is separated by column chromatography gradient elution method, wherein the fineness of silica gel is 200-300 me...
Embodiment 2
[0039] This example illustrates the preparation of 1-(3-amino-1,2,4-triazol)-yl-3,3-dimethyl-2-butanone.
[0040] 0.8mmol of 3-methyl-1,2,4-triazole was mixed with an equivalent amount of pyridine, and pyridine was used as a catalyst and an acid-binding agent; 50mL of acetonitrile was added as a reaction solvent; 0.6mmol of 1-tert-butyl-2 - Chloro-ethanone and 10ml of acetonitrile are miscible and diluted, and the whole is added to the above reaction system; microwave radiation is 4min, and the radiation power is 200W; after the reaction is completed, cool to room temperature, concentrate under reduced pressure to remove the acetonitrile solvent, dissolve the product in ethanol, and filter , and wash the solid 3 times with ethanol, each time the amount of ethanol is not more than 20mL; the filtrate is combined and concentrated to about 10mL; the concentrated product is separated by column chromatography gradient elution method, wherein the silica gel fineness is 200-300 mesh, w...
Embodiment 3
[0042] This example illustrates the preparation of 1-(3-amino-1,2,4-triazol)-yl-3,3-dimethyl-2-butanone.
[0043]1.19mmol of 3-methyl-1,2,4-triazole was mixed with an equivalent amount of pyridine, and pyridine was used as a catalyst and an acid-binding agent; 10mL of acetonitrile was added as a reaction solvent; 0.99mmol of 1-tert-butyl-2 - Chloro-ethanone and 15ml of acetonitrile are miscible and diluted, and the whole is added to the above reaction system; microwave radiation for 3 minutes, and the radiation power is 400W; after the reaction is completed, cool to room temperature, concentrate under reduced pressure to remove the acetonitrile solvent, dissolve the product in ethanol, and filter , and wash the solid 3 times with ethanol, each time the amount of ethanol is not more than 20mL; the filtrate is combined and concentrated to about 5mL; the concentrated product is separated by column chromatography gradient elution method, wherein the fineness of silica gel is 200-30...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com