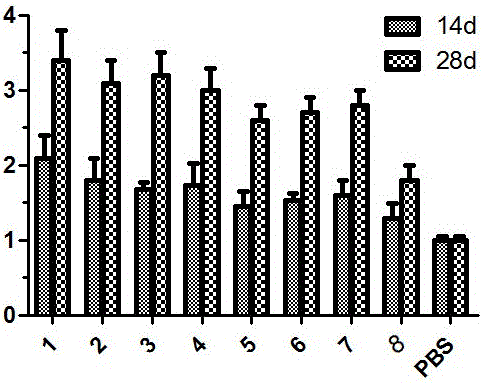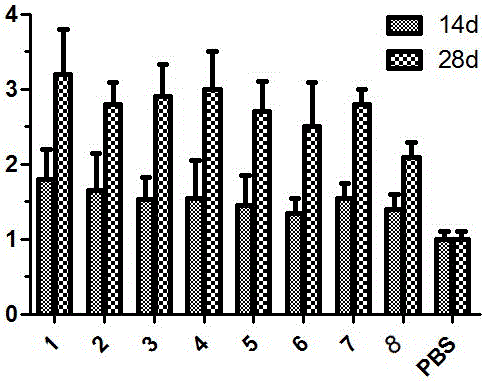Virus provided with gene cooperation element and applications of virus
An element and gene technology, which is applied to viruses with gene synergistic elements and their application fields, can solve the problem of low efficacy of the virus, and achieve the effect of obvious advantages, optimized structure, and good disease prevention or treatment effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
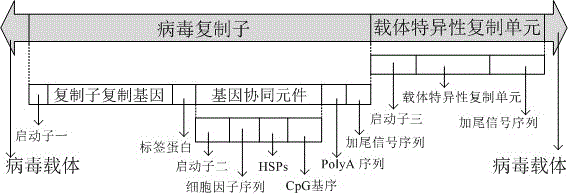
[0041] Viruses with gene coordination elements consist of promoter one, replicon replication gene, tag protein, promoter two, cytokine sequence, heat shock protein sequence HSPs, CpG motif, PolyA sequence, tailing signal sequence, Promoter three, vector replication gene, tailing signal sequence, which are inserted into the viral vector together, are called Ad-SFV-ABC-E1A, and its structure is as follows figure 1 Shown.
[0042] Among them, the virus vector uses adenovirus pShuttle-CMV vector, and uses BglII-HindIII for restriction digestion. The gene sequence at both ends of the insertion site after restriction digestion is as follows:
[0043] 5'-...CATCATCAATATTATACCTTATTTTGGATTGAAGCCAATATGATAATGAGGGGGTTATGGAGTTTGTCAC-3';
[0044] 5'-AACGCGGATCTGGGCGTGGTTAAGGGTGGGAAAGAATATATAAGGT GGGGGTCTTATG...-3'.
[0045] In this embodiment, the promoter one uses mEF1 promoter-BglII, the replicon replication gene is composed of the pSFV gene sequence obtained after the alphavirus replicon is dig...
Embodiment 2
[0055] The difference between this embodiment and embodiment 1 lies in: this embodiment removes part of the gene sequence from the Ad-SFV-ABC-E1A described in embodiment 1, and the specific settings are as follows:
[0056] Tumor drug group 1: Ad-SFV-ABC-E1A.
[0057] Tumor Drug Group 2: Remove the CpG motif on the basis of Ad-SFV-ABC-E1A, the CpG motif is a CpG island, and the sequence of the CpG island is shown in SEQ ID NO: 2;
[0058] Oncology drug group 3: On the basis of Ad-SFV-ABC-E1A, the heat shock protein sequence HSPs are removed, and the heat shock protein sequence HSPs is Mycobacterium bovis HSP70. The sequence of M. tuberculosis HSP70 is as SEQ ID NO: 3 shown;
[0059] Tumor drug group 4: On the basis of Ad-SFV-ABC-E1A, the cytokine sequence is removed, the cytokine sequence is mGM-CSF, and the sequence of mGM-CSF is shown in SEQ ID NO: 4;
[0060] Oncology drug group 5: remove the heat shock protein sequence HSPs and CpG motifs on the basis of Ad-SFV-ABC-E1A;
[0061] Tum...
Embodiment 3
[0075] The difference between this embodiment and embodiment 1 is that the virus of this embodiment does not include a vector-specific replication unit, and the GFP sequence is replaced with the mouse parvovirus MVM-VP2. The specific sequence of the MVM-VP2 in this embodiment The structure is shown in SEQ ID NO: 5, and the gene sequence of the vector-specific replication unit is shown in SEQ ID NO: 6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com