Application of pentacyclic triterpene compounds to preparation of medicine for treating adiposis
A technology of pentacyclic triterpenes and compounds, which is applied in the field of obesity treatment drugs, can solve problems such as many side effects, achieve strong inhibitory effect, inhibit absorption capacity, and reduce weight gain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of tablets: Mix 60g pedicel glucoside and 20g pregelatinized starch evenly, add 50% ethanol as binder, make soft material, pass through a 20 mesh sieve, make granules, and place the obtained wet granules at 75°C Dry in an oven for 2 hours, add appropriate amount of micropowder silica gel and magnesium stearate to the granules, mix evenly, press into tablets and pack separately.
Embodiment 2
[0021] Lipase inhibition rate determination experiment: Take 100 μl of porcine pancreatic lipase (PL) solution (5 mg / mL) and add 20 μL of different concentrations of the sample solution to be tested, use Tris-HCl buffer solution with pH=7.4 to make the volume to 900 μl, mix well, Incubate at 37°C for 5 minutes, then add 100 μL of p-nitrophenylbutyrate (p-NPB) solution (10mol / L), shake well, transfer 200 μL to a 96-well microplate quickly, and use a microplate reader to determine its The absorbance at 410nm is read every 1 minute and measured at least 5 times (1 to 15 minutes). The same operation was repeated 3 times for the determination of each concentration in each group, and the absorbance was averaged. Take time as the abscissa, and take the difference between the absorbance in the experimental group and the absorbance of the corresponding blank group as the ordinate, and calculate the change rate K of the absorbance difference with time, and calculate the inhibition rate ...
Embodiment 3
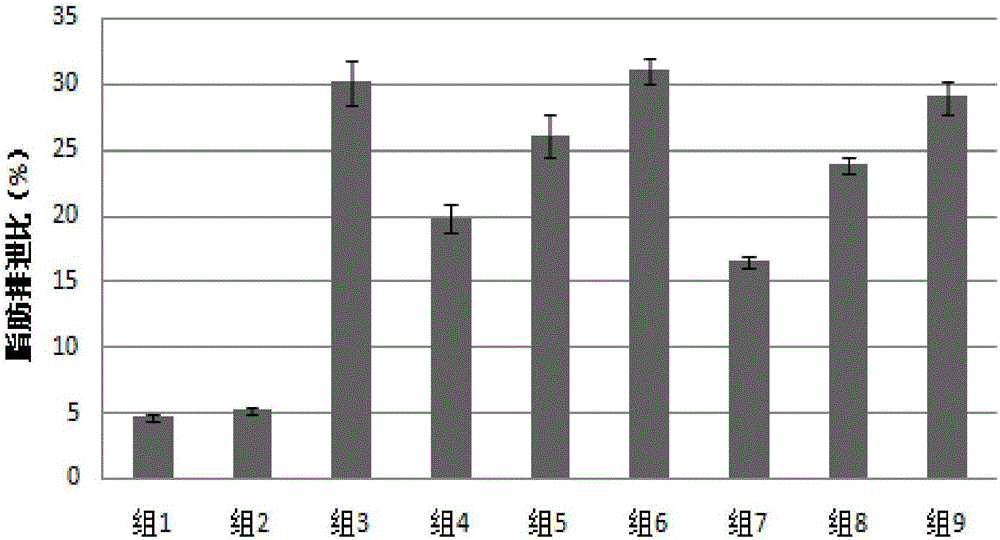
[0026] Effects of similar compounds with a pentacyclic triterpene as the parent nucleus on obesity induced by high-fat diet: 54 clean-grade Wistar rats, aged 3 to 4 months, weighing 180g-200g, male, purchased from Guangdong Experimental Animal Center. The fat energy content of the basic nutrition feed is 10%, and the fat energy content of the high-fat nutrition feed is 45%. After one week of adaptive feeding with the basic nutritional feed, the mice were randomly divided into 9 groups (n=6), and the grouping situation was as follows:
[0027] Group 1: fed with basic nutrient feed for 12 weeks, as the normal group.
[0028] Group 2: fed with high-fat nutrient feed for 12 weeks, and in the last 4 weeks, the same volume of 0.5% sodium hydroxymethyl cellulose solution was given by intragastric administration every day, as a blank control group.
[0029] Group 3: fed with high-fat nutrient feed for 12 weeks, of which 10mg·kg was given daily for the last 4 weeks -1 Orlistat (orli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com