Crystal form alpha of vonoprazan fumarate and the preparation method thereof
A technology for vornorazan fumarate and crystal form, which is applied in the preparation of the new crystal form, in the field of vornorazan fumarate crystal form α, can solve the problem of difficult to remove impurities, difficult to take into account yield and purity, Problems such as the purification method of vonoprazan fumarate are not provided to achieve the effects of high purity, easy industrial preparation and good controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example
[0025] Refer to the method of CN201080018114.9 Example 5 to prepare the crude product of vonoprazan fumarate
[0026] To a nitrogen purged flask were added N,N-dimethylacetamide (108 ml) and sodium borohydride (3.06 g, 81.74 mmol), and the mixture was dissolved (Solution A). Into another nitrogen-purged flask was added 5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carbaldehyde (60.0 g, 181.64 mmol) and methanol ( 300ml), then a 40% solution of methylamine in methanol (18.34g, 236.13mmol) was added dropwise at room temperature. The mixture was further stirred for 30 minutes at an internal temperature of 20-30°C. The internal temperature was cooled to -10°C, and then the previously prepared solution A was added dropwise at the internal temperature not exceeding 0°C. N,N-Dimethylacetamide (12 ml) was added, and the mixture was stirred at an internal temperature of -10-0°C for 1 hour. 1N HCl (360 ml) was added dropwise at an internal temperature not exceeding 20°C, a...
Embodiment 1
[0028] Preparation of Vonorazan Fumarate Form α
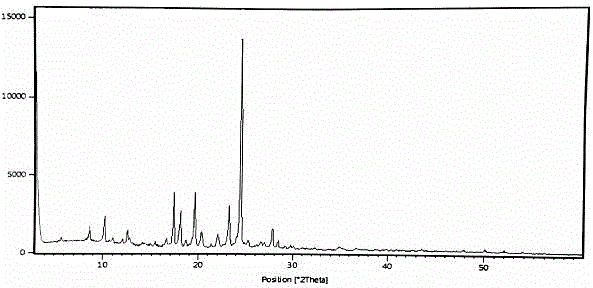
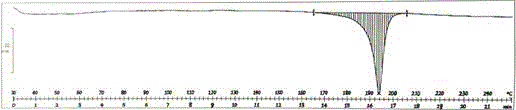
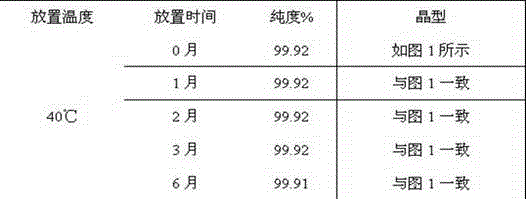
[0029] Add 10 g of vonoprazan fumaric acid crude product to 100 g of ethylene glycol monomethyl ether, heat to 60 ° C to dissolve, then add 300 g of purified water, stir and react at room temperature for 1 hour, filter, wash the filter cake with purified water, and dry to obtain White solid crystal 9.2g, yield 92%, HPLC purity 99.92%; After determination, its X-RPD spectrum is as follows figure 1 As shown, its DSC-TGA spectrum is as figure 2 shown.
Embodiment 2
[0031] Preparation of Vonorazan Fumarate Form α
[0032] Add 10 g of vonoprazan fumaric acid crude product to 30 g of ethylene glycol monomethyl ether, heat to reflux to dissolve, then add 90 g of purified water, stir and react at room temperature for 1 hour, filter, wash the filter cake with purified water, and dry to obtain white 9.3 g of solid crystals, yield 93%, HPLC purity 99.89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com