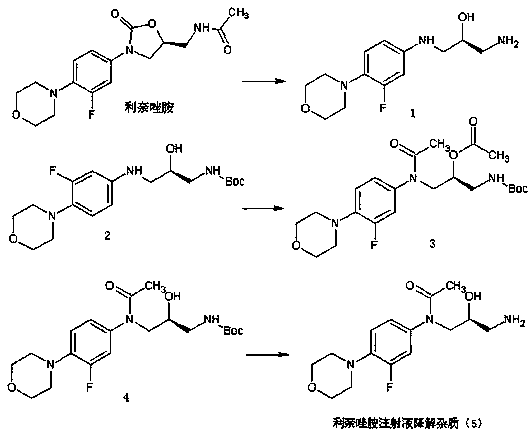
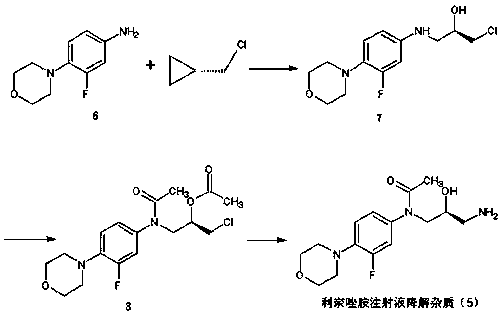
Preparation method of linezolid injection for degrading impurities
A technology for linezolid and injection, which is applied in the field of preparation of linezolid injection impurities, which can solve problems such as difficulty in refining and affecting the purity of the final product, and achieve the effects of stable quality, mild reaction conditions, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Preparation of (2R)-1-chloro-3-{[3-fluoro-4-(morpholin-4-yl)phenyl]amino}-propan-2-ol (7)
[0020] Add 10g of intermediate (6) to a 250mL three-neck flask, then add 100mL of acetonitrile, dissolve at room temperature, then add 6.9g of (S)-epichlorohydrin, and heat up to 50°C for reaction. After 5 hours, the solvent was distilled off, 100 mL of dichloromethane was added, and washed with 3×50 mL of water. The organic layer was dried over anhydrous sodium sulfate and concentrated to obtain 10.4 g of oily intermediate (7) with a purity of 96.2% and a yield of 71%.
Embodiment 2
[0021] Example 2: Preparation of (2R)-1-{acetyl[3-fluoro-4-(morpholin-4-yl)phenyl]amino}-3-chloropropan-2-yl acetate (8)
[0022] Dissolve 10.4g of intermediate (7) in 42mL of dry dichloromethane, add it to a 100mL three-necked flask, then add 8.7g of triethylamine, cool to 0-5°C, add 6.8g of acetyl chloride dropwise, and finish dropping within half an hour. Then it was raised to 20-25°C for 2 hours, and the reaction solution was quenched by adding 40 mL of ice water. The organic layer was washed with 3×50 mL of water, dried over anhydrous sodium sulfate, and concentrated. 11.0 g of oil was obtained with a purity of 92.5% and a yield of 82%.
Embodiment 3
[0023] Example 3: Preparation of (2R)-1-{acetyl[3-fluoro-4-(morpholin-4-yl)phenyl]amino}-3-chloropropan-2-yl acetate (8)
[0024] Dissolve 10.4g of intermediate (7) in 42mL of dry dichloromethane, add it to a 100mL three-necked flask, add 6.8g of pyridine, cool to 5-10°C, add 6.8g of acetyl chloride dropwise, and finish dropping within half an hour. Then it was raised to 25-30°C for 2 hours, and the reaction solution was quenched by adding 40 mL of ice water. The organic layer was washed with 3×50 mL of water, dried over anhydrous sodium sulfate, and concentrated. 11.3 g of oil was obtained with a purity of 93.6% and a yield of 84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com