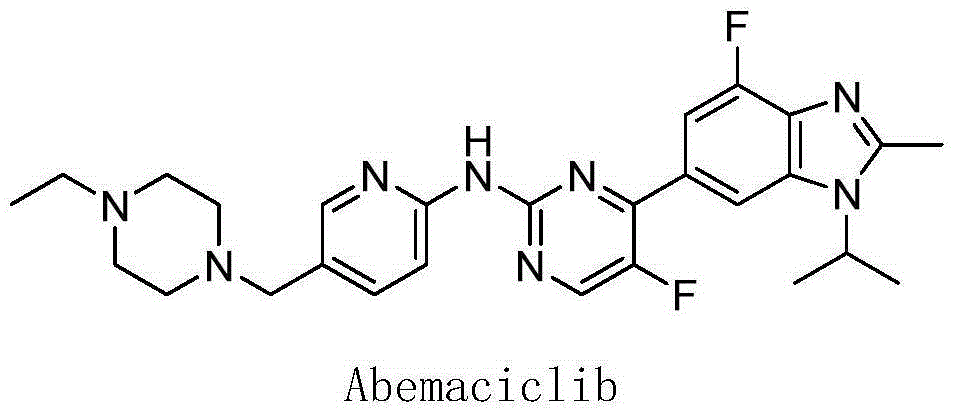
Preparation method of Abemaciclib intermediate
A compound and selected technology, applied in the field of medicine and chemical industry, can solve the problems of low yield, harsh reaction conditions, difficult reaction and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
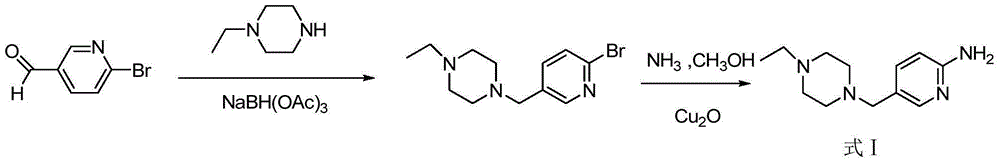
[0075] Example 1 Preparation of 5-(4-ethylpiperazine-1-carbonyl)-2-nitropyridine (compound of formula III)
[0076] Add 6-nitronicotinic acid (10g, 59.5mmol), N-ethylpiperazine (8.15g, 71.4mmol), N,N-diisopropylethylamine (15.37g, 119.0mmol) and 500ml reactor DMF (100ml). The temperature of the reaction system was lowered to 0°C while stirring. PyBOP (34.05 g, 65.5 mmol) was slowly added to the reaction solution, and the reaction solution slowly dissolved. After PyBOP was added, the reaction was continued for 30 minutes, and the temperature of the reaction solution was raised to 25°C. Stop the reaction after reacting for 2 hours, slowly add 500ml of water to the reaction system, separate out the solid, add ethyl acetate and extract three times (each 200ml), the organic phase of the separated gained is washed with saturated saline, dried over anhydrous sodium sulfate, filtered, Concentrate in vacuo and purify by column chromatography (200-300 mesh silica gel, dichloromethane...
Embodiment 2
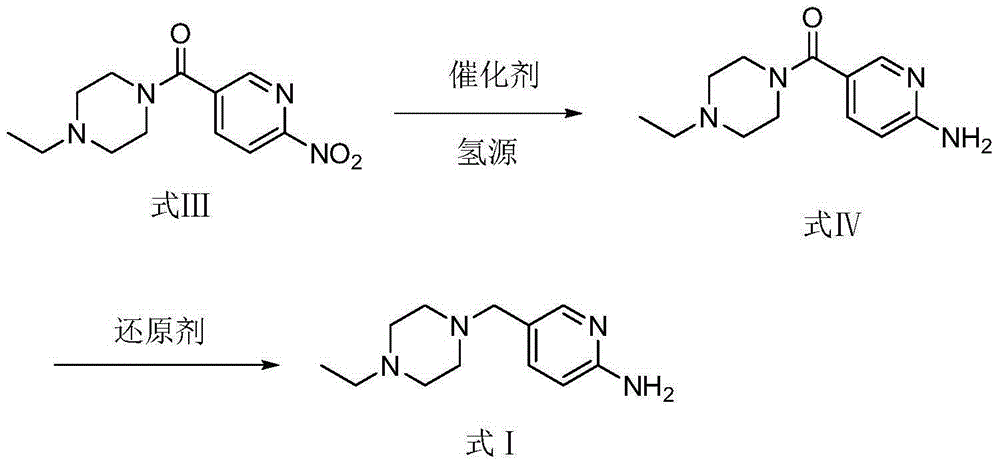
[0080] Example 2 Preparation of 5-(4-ethylpiperazine-1-carbonyl)pyridin-2-amine (compound of formula IV)
[0081] Add the compound of formula III (10 g, 37.84 mmol), 10% Pd / C (2.5 g, containing 50% water, 1.2 mmol), methanol (250 ml) into a 100 ml reactor. Under stirring, the temperature of the reaction system was raised to 50°C for hydrogenation under normal pressure. After 2 hours of reaction, stop the reaction, filter, wash the filter cake with methanol (10ml), and concentrate the filtrate to obtain 7.5g of the compound of formula IV, yield: 84.3%.
[0082] ESI-MS[M+H] + :235.1537.
[0083] 1 H NMR (300MHz, DMSO-d6): δ7.99 (d, J = 1.5Hz, 1H), 7.42 (dd, J = 8.5, 1.5Hz, 1H), 6.43 (d, J = 8.5Hz, 1H), 6.36 (s, 2H), 3.49 (m, 4H), 2.30-2.50 (m, 6H), 0.99 (t, J=7.2Hz, 3H).
[0084] 13 C NMR (75 MHz, DMSO-d6): δ 167.87, 160.44, 147.83, 136.71, 118.82, 106.71, 52.29, 51.40, 44.77, 11.78.
Embodiment 3
[0085] Example 3 Preparation of 5-(4-ethyl-piperazin-1-ylmethyl)-pyridin-2-ylamine (compound of formula I)
[0086] Tetrahydrofuran (50ml) was added into a 100ml reactor, and the temperature of the reaction system was lowered to 0°C under the protection of nitrogen. Lithium aluminum hydride (3.1g, 85.2mmol) was first added to tetrahydrofuran, and then the compound of formula IV (5.0g, 21.3mmol ), stop the reaction after reacting at 0°C for 3 hours, slowly add 1N sodium hydroxide dropwise at 0°C, stir, and no gas is released. Dichloromethane was extracted three times (50ml each time). The separated organic phase was washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered, concentrated in vacuo and purified by column chromatography (200-300 mesh silica gel, dichloromethane:methanol=200:1, 150:1, 100 : 1 gradient elution, collect single product spot eluent, concentrate), obtain solid 4.1g, yield: 87.2%, HPLC purity 97.2% (area normalization ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com