Compounds useful as sglt2 inhibitors and their preparation and use
A technology of compounds and ketone compounds, applied to compounds that can be used as SGLT2 inhibitors and the fields of their preparation and use, can solve weight gain, hypoglycemia and gastrointestinal discomfort, involve many targets, and complex pathogenesis of type 2 diabetes And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
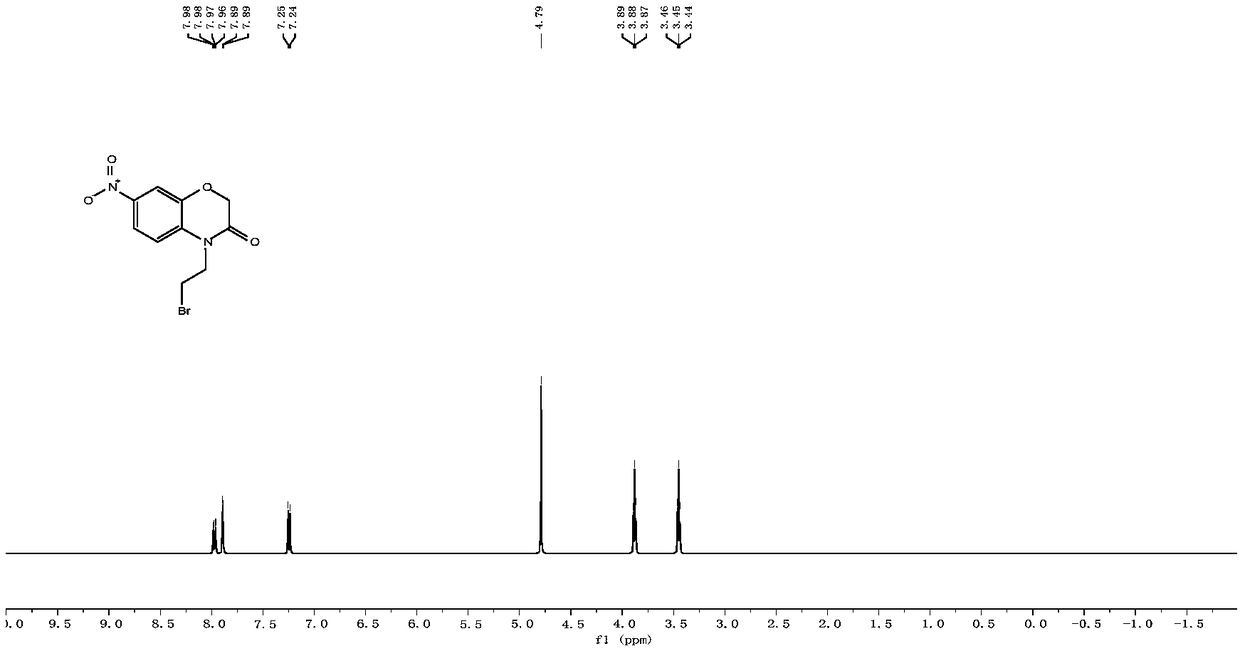
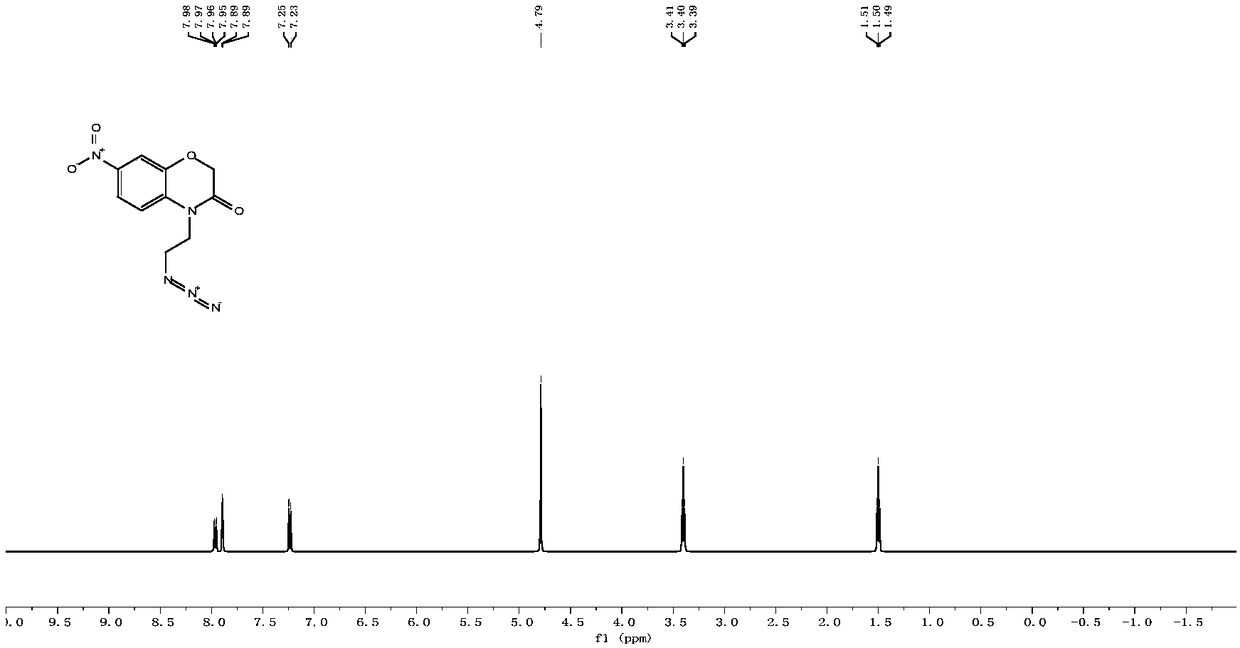
[0078] Nitric acid ((1-(2-(3-oxo-2,3-dihydro-4H-benzo[b][1,4]oxazin-4-yl)ethyl)-1H-1,2, Preparation of 3-triazol-4-yl)methylcarbonic anhydride.
[0079] Step 1: Preparation of intermediate 2H-benzo[b][1,4]oxazin-3(4H)-one
[0080]
[0081] 2-Aminophenol (5.7g, 52.0mmol) was dissolved in 100mL acetonitrile and pre-cooled for 10min, TEBA (1.2g, 5.2mmol) and NaHCO were added at 0°C 3 (8.7 g, 103.6 mmol) and kept stirring. Then a solution of 2-chloroacetyl chloride (4.9 mL, 62.4 mmol) in 20 mL of acetonitrile was slowly added dropwise. After the dropwise addition was completed, keep stirring at 0°C for 40 minutes, then transfer to a 55°C oil bath for stirring and reacting for 8 hours, concentrate the reaction solution with a rotary evaporator, then recrystallize with ethanol:water (1:1) mixture, suction filter, vacuum After drying, 5.3 g of light brown solid 2H-benzo[b][1,4]oxazin-3(4H)-one was obtained, with a yield of 68.4%. m.p.: 171-173°C; 1 H NMR (400MHz, CDCl 3 )δ(p...
Embodiment 1-15
[0095] According to the general preparation method, 2H-benzo[b][1,4]oxazin-3(4H)-one SGLT2 inhibitors listed in Table 1 were synthesized using different substituted forms of 2-aminophenol as raw materials .
[0096] Table 1 2H-Benzo[b][1,4]oxazin-3(4H)-one compounds
[0097]
Embodiment 1~15
[0098] Embodiment 1~15 1 H NMR and MALDI-TOF-MS data
[0099] Nitric acid ((1-(2-(3-oxo-2,3-dihydro-4H-benzo[b][1,4]oxazin-4-yl)ethyl)-1H-1,2, 3-triazol-4-yl)methylcarbonic acid)anhydride (Example 1): white solid, yield 35.3%, m.p.: 67.5-68.4°C. 1 H NMR (400MHz, DMSO-d 6 )δ (ppm): 8.34 (s, 1H), 7.06 (ddd, J = 7.5, 6.2, 2.9Hz, 1H), 7.06-6.92 (m, 3H), 5.42 (s, 2H), 4.79 (s, 2H ), 4.50(t, J=5.7Hz, 2H), 3.86(t, J=5.7Hz, 2H).MALDI-TOF: m / z 363([M+H] + ).
[0100] Nitric acid ((1-(3-(3-oxo-2,3-dihydro-4H-benzo[b][1,4]oxazin-4-yl)propyl)-1H-1,2, 3-triazol-4-yl)methylcarbonic acid) anhydride (Example 2): white solid, yield 39.3%, m.p.: 80.3-81.7°C. 1 H NMR (400MHz, DMSO-d 6)δ(ppm):7.64(s,1H),7.11-6.95(m,2H),6.99-6.91(m,2H),5.42(s,2H),4.79(s,2H),4.42(t,J =7.4Hz, 2H), 4.30(t, J=7.7Hz, 2H), 2.13(p, J=7.6Hz, 2H).MALDI-TOF: m / z 377([M+H] + ).
[0101] Nitric acid ((1-(4-(3-oxo-2,3-dihydro-4H-benzo[b][1,4]oxazin-4-yl)butyl)-1H-1,2, 3-triazol-4-yl)methylcarbonic anhydride (Example...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com