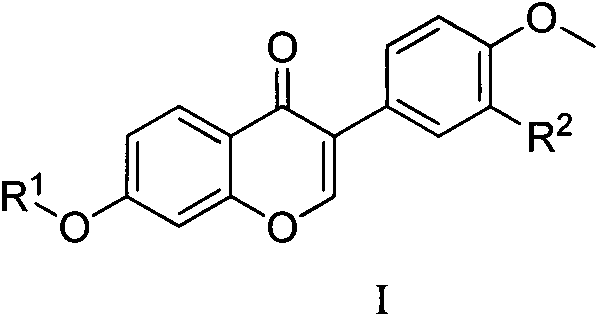
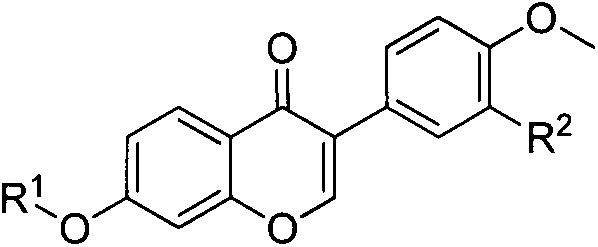
Formononetin fatty ether derivatives and preparation methods and medical application thereof
A fatty acid and fatty alkoxy technology, applied in the field of medicinal chemistry, can solve the problems of fast metabolism, easy hydrolysis and unstable ester bonds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
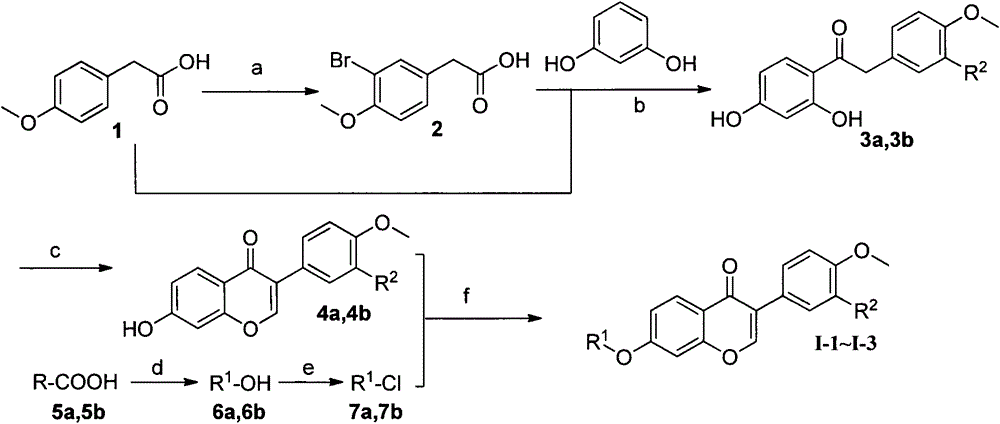
preparation example Construction
[0070] The preparation of some compounds is carried out as follows:
[0071] Melting point was determined by XRC-1 micro melting point apparatus (the thermometer was not calibrated), IR was determined by Nicolet iS10 Fourier transform infrared spectrometer (KBr tablet), 1 H-NMR nuclear magnetic resonance was measured by a Bruker AV300 (300MHz) nuclear magnetic resonance instrument (TMS was an internal standard), and the mass spectra were determined by a Shimadzu GC / MS-QP2010 mass spectrometer (EI-MS), Agilent1 100LC-MSD-Trap / SL mass spectrometer (ESI-MS) determination.
[0072] The silica gel used for column chromatography is 100-200 mesh, 200-300 mesh or 300-400 mesh silica gel (Qingdao Ocean Chemical Factory), and the eluent is petroleum ether-ethyl acetate system or chloroform-methanol system. Thin-layer chromatography (TLC) uses a GF254 thin-layer chromatography plate (Yantai Jiangyou Silica Gel Development Co., Ltd.); the TLC development system is a petroleum ether-ethy...
Embodiment 1
[0074] Preparation of 7-hydroxy-3-(4-methoxyphenyl)-4H-chromen-4-one (3a)
[0075] In the first reaction flask, add resorcinol (0.72g, 6.5mmol), p-methoxyphenylacetic acid (1; 1.0g, 6.0mmol), BF 3 / Et 2 O (10ml), stirred at 75°C for 90min. After the reaction was completed, cool to below 10°C, and slowly add DMF (10 mL) under stirring. In the second reaction bottle, add DMF (20mL), cool to below 10°C, add PCl in batches 5 (2.0g, 9mmol), stirred at 55°C for 30min. After the reaction is over, cool to below 10°C, slowly add to the first reaction bottle, and react at room temperature for 1h. After the reaction is completed, pour the reaction solution into hot dilute hydrochloric acid (0.1N) under stirring, and a solid precipitates, which is suction filtered, washed with water, and dried. The crude product was recrystallized from methanol to obtain 1.0 g of the product with a yield of 62.5%. mp 256-258°C; MS (ESI): m / z = 269 [M+H]+.
Embodiment 2
[0077] Preparation of 3-(4-methoxyphenyl)-7-(3-(4-methoxyphenyl)propoxy)-4H-chromen-4-one (4a)
[0078] Add 7-hydroxy-3-(4-methoxyphenyl)-4H-benzopyran-4-one (3a, 5g, 1.3mmol), K 2 CO 3 (1.0g), KI (0.2g), 1-(3-chloropropyl)-4-methoxybenzene (1.65mmol), DMF (10mL), stirred at 75°C for 5h. After the reaction was completed, cool to room temperature, pour the reaction liquid into ice water under stirring, and precipitate a solid, suction filter, wash with water, and dry to obtain a crude product. Column chromatography (petroleum ether / ethyl acetate: 5 / 1, V / V) gave 450 mg of a white solid with a yield of 83.2%. mp 178-180°C; IR (cm -1 ): 3416, 2909, 2838, 1631, 1609, 1566, 1515, 1445, 1250, 1183, 1024, 827, 812; 1 H-NMR (CDCl 3 , 300MHz); δ8.21(d, 1H, J=8.82Hz, H-5), 7.91(s, 1H, H-2), 7.50(d, 2H, J=8.28Hz, H-2′, H -6'), 7.13(d, 2H, J=8.37Hz, H-2", H-6"), 6.98(m, 3H, Ar-H), 6.83(m, 3H, Ar-H), 4.04 (t, 2H, J=6.06Hz, -OCH 2 -), 3.84 (m, 3H, -OCH 3), 3.79 (m, 3H, -OCH 3 ), 2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com